Background: The metabolic syndrome and non-alcoholic fatty liver disease are increasing at alarming rates.
Aims: To determine the effect of HMG-CoA reductase inhibitors (statins) on elevated liver enzymes in patients with hyperlipidemia.
Patients: Patients with AST above 60 U/L prior to or during treatment with statin therapy at a quaternary care lipid clinic were reviewed.
Methods: A retrospective analysis was conducted. Patients were separated into two groups: Group 1 – elevated AST prior to statin therapy; and Group 2 – elevated AST during statin therapy.
Results: Forty six patients with one or more measurements of AST >60 U/L remained after exclusion criteria were applied. Ten of 13 (77%) group 1 patients had reduced AST levels after initiation of statin therapy. Thirty two of 33 patients (97%) in group 2 had transient AST elevations while on statin therapy; one patient had persistently elevated AST after initiation of treatment. There were no significant adverse events reported.
Conclusion: Use of HMG-CoA reductase inhibitors in patients with elevated AST resulted in normalization of AST levels. HMG-CoA reductase inhibitors were safe in patients with mildly elevated AST. This may translate to use of HMG-CoA reductase inhibitors in diseases such as non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.
Work was performed at St Paul’s Hospital, Vancouver, British Columbia, Canada.
IntroductionNon-alcoholic steatohepatitis (NASH) represents the late stage of a continuum of changes to hepatic structure and content from fatty infiltration (or non-alcoholic fatty liver disease – NAFLD) to frank fibrosis and on occasion cirrhosis.1 Frequent associations with the disease include impaired glucose tolerance (an independent risk factor for death in NAFLD),2 type 2 diabetes mellitus, lipid abnormalities and the constellation of the above that encompasses the metabolic syndrome.3 Estimates of the prevalence in various countries range from 10-24%.4 A high variability of this number is accounted for by the invasive measure of liver biopsy needed for definitive diagnosis.
North American obesity rates are increasing at alarming rate, along with the prevalence of NAFLD. In Canada, obesity (BMI ≥ 30 kg/m2) rates have increased from 15% in 1992 to 23% 2004.5
Given the overlap of diseases in the metabolic syndrome, several putative targets for treatment have been investigated. Weight loss and exercise are the cornerstone of care in all patients regardless of the presence of NASH. Treatment of hyperlipidemia with HMG-CoA reductase inhibitors (statins)6-8 and fibrates9,10 has also been investigated. Studies have proven that statins are safe in patients with mild to moderate liver enzyme elevations.11
Previous studies have shown positive results following treatment with HMG-CoA reductase inhibitors in patients with NAFLD. Most recently, Gomez-Dominguez and colleagues8 published a pilot study of atorvastatin treatment in dyslipidemic patients with NAFLD. In this study, baseline AST was 46.7 U/L. After six months of treatment with atorvastatin, 36.3% of patients had normal AST levels. A further 20% of patients had normal levels after 12 months of treatment.8 Another study showed a decrease of AST from 68 to 46 U/L in 28 patients with hypercholesterolemia treated with atorvastatin for 24 weeks. As well, liver echopattern normalized by the end of treatment in 61% of patients.7 A third study showed a significant reduction of AST as well as an increase in liver densities in 27 hyperlipidemic NASH patients treated with atorvastatin for six months.6 None of these studies reported any adverse effects with treatment.
Our primary objective was to determine the effect of statins on elevated liver enzymes in patients with hyperlipidemia. In order to obtain data from a large population of dyslipidemic patients, we chose the Lipid Clinic Database of a quaternary care centre to review liver biochemical abnormalities. We hypothesized that optimization of lipid profile in patients with abnormal liver enzymes consistent with NAFLD will result in stabilization or resolution of the metabolic disturbances of the liver found in NAFLD. Our secondary aim was to determine safety of statin therapy in patients with elevated AST.
Materials and methodsCharts at the St. Paul’s Hospital Healthy Heart Program (Lipid Clinic Database) were reviewed for liver biochemistry abnormalities. This database is designed to track patients followed at the Lipid Clinic. Those patients treated from 1993 to 2004 with AST above 60 U/L on one or more measurement were identified. This AST level corresponds to 1.5 times the upper limit of normal, and in the absence of other liver pathology is strongly suggestive of NAFLD.12
Baseline characteristics including patient demographics, AST and lipid levels, alcohol consumption, presence of type 2 diabetes mellitus, and duration of statin therapy were collected. Baseline laboratory investigations in the database included cholesterol panel and usually AST, but no other liver markers. Personal demographic data, including alcohol consumption was entered from forms filled out by the patients on their first clinic visit. If alcohol consumption was missing from the database, the paper charts were reviewed.
Patients were separated into two groups: either Group 1 – patients with elevated AST on commencement of statin therapy, or Group 2 – patients with normal AST on commencement of statin therapy with elevated AST during statin therapy. AST, cholesterol and triglyceride levels were compared prior to therapy and at the end of follow up to determine biochemical response to treatment with statins.
Exclusion criteria: patients with alcohol consumption or greater than one and two drinks per day for women and men, respectively; and patients with incomplete or inaccessible baseline or follow up data.
The study was approved by the University of British Columbia Clinical Research Ethics Board.
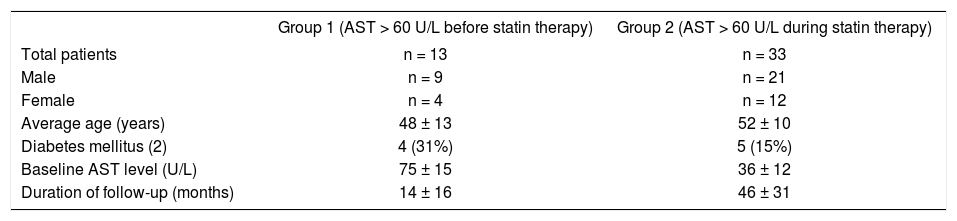
ResultsA total of 516 patients with one or more measurements of AST > 60 U/L were identified. Forty six patients remained after exclusion criteria were applied. The majority of exclusions were due to excessive alcohol consumption, or incomplete database records (i.e. no follow up AST levels or alcohol consumption not indicated). Baseline characteristics of the two groups are outlined in Table I. Four of 13 (31%) Group 1 patients had type 2 diabetes mellitus. The duration of follow-up for Group 1 and 2 was 14 and 46 months, respectively.
Baseline characteristics.
| Group 1 (AST > 60 U/L before statin therapy) | Group 2 (AST > 60 U/L during statin therapy) | |
|---|---|---|
| Total patients | n = 13 | n = 33 |
| Male | n = 9 | n = 21 |
| Female | n = 4 | n = 12 |
| Average age (years) | 48 ± 13 | 52 ± 10 |
| Diabetes mellitus (2) | 4 (31%) | 5 (15%) |
| Baseline AST level (U/L) | 75 ± 15 | 36 ± 12 |
| Duration of follow-up (months) | 14 ± 16 | 46 ± 31 |
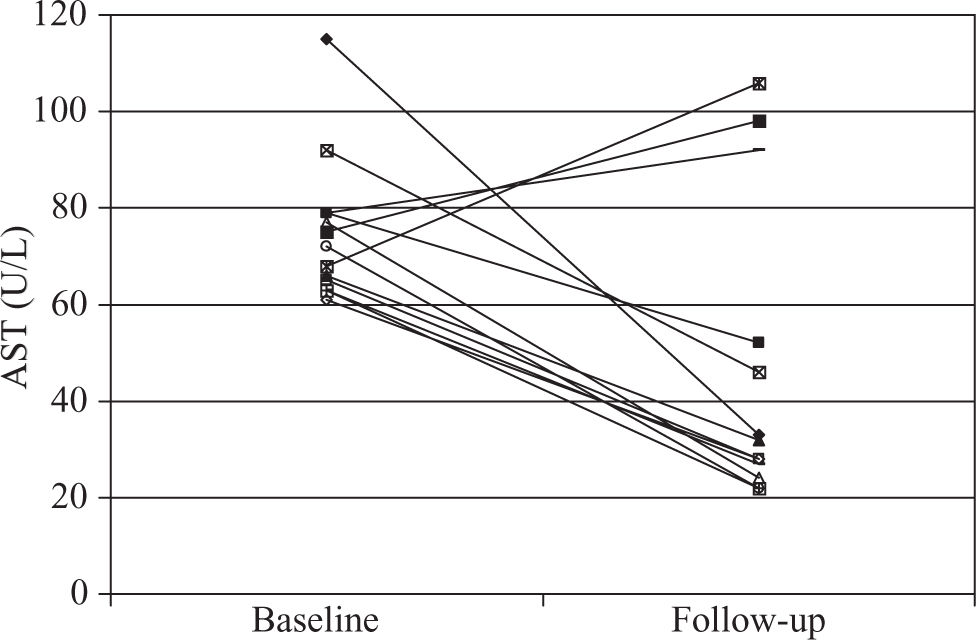
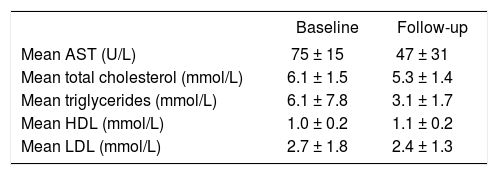
Before treatment, average AST level in Group 1 was 75 U/L which is 1.9 times the upper limit of normal. Ten of 13 (77%) Group 1 patients had reduced AST levels below 60 U/L after initiation of statin therapy; eight of these had complete normalization of levels(Figure 1). Average AST at final follow-up was 47 U/L. Total cholesterol levels decreased by 13% and triglyceride levels decreased by 49%. HDL increased by 0.1 mmol/L and LDL levels decreased by 0.3 mmol/L (Table II).
A second group of patients with normal AST prior to statin therapy was used to evaluate safety. Thirty three patients who developed an AST > 1.5 times upper limit of normal during therapy were identified. Thirty two of 33 patients (97%) in group 2 had only transient AST elevations ranging from 62 to 264 U/L. One of these patients AST rapidly increased to 974 U/L while taking Cervistatin which was then discontinued. The AST decreased to 682 U/L two days later and then was normal at 38 U/L after five months. One patient had persistently elevated AST after initiation of treatment. There were no other significant adverse events reported in this group.
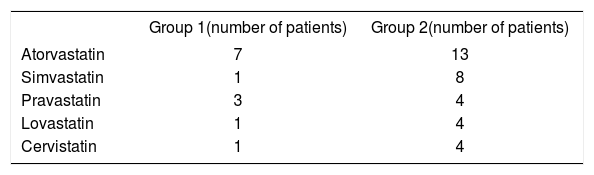
Type of statins was variable (Table 3). Atorvastatin and Simvastatin were most frequently used (62% and 64%, respectively for Group 1 and 2).
DiscussionNon-alcoholic fatty liver disease (NAFLD) is an increasingly recognized cause of chronic liver disease and affects up to one third of the general population in the United States.2 Given the association of NAFLD with metabolic syndrome and the increasing prevalence of obesity and diabetes mellitus in Western countries, effective and safe therapies for NAFLD must be established.
Our Group 1 patients had average AST levels of 1.9 times the upper limit of normal, along with hyperlipidemia and hypertriglyceridemia. In addition, 31% had type II diabetes. This combination of abnormalities is strongly suggestive of NAFLD.12 Given the nature of our database (i.e. primarily designed to track Lipid Clinic patients); we were not able to definitively rule out liver pathology other than alcohol use. In this group of patients, AST levels decreased below 60 U/L in 77% of patients; eight had complete normalization of their AST level. Furthermore, the total cholesterol and triglyceride levels improved with treatment, suggesting that optimization of lipid profile in patients with abnormal liver enzymes consistent with NAFLD results in resolution of the metabolic disturbances of the liver found in NAFLD.
The second group of patients was investigated to determine safety of continued statin therapy in patients with mild to moderate AST elevations. Ninety seven percent of patients had only transient elevations in AST. No significant adverse events were recorded in Group 1 patients. One patient in Group 2 had an AST elevation to 975 U/L which was contributed to Cervistatin. This is further confirmation that HMG-CoA reductase inhibitors are safe in patients with mild to moderately elevated AST.
There are controversies and unresolved questions concerning the use of HMG-CoA reductase inhibitors in patients with liver disease. The most important complication of statin therapy is rhabdomyoloysis.13 Patients with steatosis and NAFLD have been shown to have decreased hepatic CP3A expression and activity.14 As well, patients with cirrhosis have higher serum concentrations of statins.15 This may contribute to patients with liver disease having higher rates of hepatotoxicity and rhabdomyolysis. However, Chalasani and colleagues16 investigated a large number of hyperlipidemic patients with elevated baseline liver enzymes and did not find higher risk for hepatotoxicity from statins. A few small studies investigating the use of HMG-CoA reductase inhibitors in patients with NAFLD have shown promising results;6-8 however large randomized controlled trials are required to confirm the data.
Our study has some limitations including small sample size, inability to rule out other types of liver disease, and lack of pathological diagnosis of NAFLD. However, this is common in many studies on NAFLD.
In summary, our results suggest that the use of HMG-CoA reductase inhibitors in patients with elevated AST results in normalization of AST levels. As well, these agents were found to be safe in patients with mild to moderately elevated AST. This data may translate to use of HMG-CoA reductase inhibitors in diseases such as nonalcoholic fatty liver disease and non-alcoholic steatohepatitis.