Non-alcoholic fatty liver disease (NAFLD) is an emerging cause of graft dysfunction after liver transplantation (LT) frequently related to the development of new onset diabetes after LT (NODAT). This study was undertaken to evaluate the frequencies of NODAT and NAFLD after LT, to investigate their major risk factors and the impact of de novo or recurrent NAFLD in graft function.
Material and methods119 patients submitted to LT were prospectively evaluated.
ResultsAfter 4 ± 1 years, NODAT, recurrent and de novo NAFLD were observed in 31%, 56% and 43% of the subjects, respectively. Only 3 patients had non-alcoholic steatohepatitis (NASH) without fibrosis. Other risk factors for NAFLD such as arterial hypertension (AHT), metabolic syndrome (MS), hypertriglyceridemia and obesity were seen in 51%, 50%, 35% and 24% of the subjects, respectively. In addition, insulin resistance (IR), assessed by HOMA-IR and β-cell dysfunction, determined by HOMA-β, were observed in 16% and 94% of the patients, respectively. Occurrence of NODAT was associated with male gender, higher waist circumference, higher HOMA-IR and lower HOMA-β values. No correlation was found between NAFLD and NODAT, MS, hypertriglyceridemia, obesity and HOMA-IR and HOMA-β levels.
ConclusionsNODAT, recurrent and de novo NAFLD are common after LT but are not associated with signs of graft dysfunction, possibly due to the low frequency of IR and NASH. No correlation is observed between NAFLD and NODAT, MS, hypertriglyceridemia, obesity and IR. β-cell dysfunction and diabetes, however, are seen in most of the patients, possibly due to calcineurin inhibitor toxicity.
New onset diabetes after transplantation (NODAT) is frequently observed after liver transplantation (LT) and is strongly associated with the use of immunosuppressive agents, particularly steroids and tacrolimus, and with the development of obesity and metabolic syndrome (MS).1 Its occurrence was shown to favor the development of infections, cardiovascular disease and to impair long-term survival. On the other hand, either type 2 diabetes or NODAT, were also associated with the development of non-alcoholic fatty liver disease (NAFLD).2-4
Non-alcoholic fatty liver disease comprises a large spectrum of histological abnormalities ranging from stea-tosis to steatohepatitis with or without fibrosis, cirrhosis and hepatocelular carcinoma (HCC).5 It affects roughly one third of the adult population worldwide in association with the increasing prevalence of its risk factors, such as metabolic syndrome (MS) and its major determinants, namely obesity, insulin resistance (IR), type 2 diabetes, arterial hypertension (AHT) and dyslipidemia.5,6 NAFLD with elevated liver enzymes and/or histologically-proven NASH are associated with an increased risk of progression to cirrhosis.7 Nowadays, NASH is the second leading etiology of cirrhosis among adults awaiting liver transplantation (LT).8 When compared to other indications for LT, patients transplanted for NASH have similar or even better survival, but an increased frequency of cardiovascular events and renal failure.8,9 NASH recurrence, on the other hand, has been reported to affect nearly all patients after LT, due to the increasing frequency of MS, obesity, IR, diabetes, arterial hypertension (AHT) and dyslipidemia observed in those subjects. Not surprisingly, de novo NAFLD has been described to occur in 18%-33% of the patients after LT,10 particularly related to the presence of NODAT, MS and its risk factors, but also to tac-rolimus-based immunosuppression, alcoholic cirrhosis as the primary indication for LT and pre-transplant liver graft steatosis.3,4,11-14
There are a paucity of data concerning the frequency and course of recurrent or de novo NAFLD after LT and their relation to NODAT and IR. Some studies have shown progressive fibrosis or even cirrhosis in subjects with recurrent NASH, but not in those with de novo NAFLD or NASH.15 It is also important to point out that most of the studies addressing the frequency of de novo NAFLD and its relation to MS are difficult to interpret, since all of them investigated de novo NAFLD retrospectively reviewing liver biopsies, previously obtained per protocol or for evaluation of graft dysfunction in different time periods after LT.8
The purpose of this study was to evaluate the frequency of NODAT, recurrent and de novo NAFLD after LT, the impact of NAFLD in graft function and to correlate the presence of NAFLD with NODAT, MS and its major risk factors.
Material and MethodsSubjectsAll patients that underwent LT between December 2001 and August 2014 in the Portuguese Hospital of Salvador, Bahia, Brazil, were prospectively investigated for enrollment in the present study. Exclusion criteria included only LT less than 12 months before evaluation and alcohol intake greater than 20 g per day in the previous 12 months. All subjects granted informed consent before entry. According to protocol all patients were interviewed and examined by one of the authors (A.R.A.) to assess the presence of AHT, type 2 diabetes, alcohol consumption, smoking, drug intake, presence or past history of cardiovascular disease, as well as other comorbidities after LT. Blood pressure was measured two times in all patients in the right arm after sitting down for at least 10 minutes in a quiet environment using an adjustable and calibrated sphygmomanometer. Arterial hypertension was considered in the presence of values greater than 140 mmHg and 90 mmHg, respectively for systolic and diastolic blood pressure, or drug intake for treatment of AHT. Weight and height were measured, respectively, on a standard balance and stadiometer and BMI was calculated as weight in kilograms divided by height in square meters, as previously described.16 Patients were considered as eutrophic, overweight and obese in the presence of BMI values of 20-25, 25-30, more than 30 kg/m2, respectively. Waist circumference was measured using a wrapping tape at the site of maximum circumference midway between the lower ribs and the anterior superior iliac spine. Abnormal values for abdominal waist were considered as 80 cm for women and 90 cm for men, as previously defined.16
After 12 h of fasting, on the same day of the interview, 10 mL of whole blood was collected from each patient for laboratory tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP), gammaglutamyltransferase (GGT), INR, bilirubin, creatinine, glucose, insulin, total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), triglycerides and ferritin levels using commercially available diagnostic tests. Abnormal values were considered according to reference parameters and required confirmation subsequently in another blood sample.
Diabetes was defined in the presence of fasting glucose levels > 126 mg/dL on at least two occasions or any drug intake for treatment of diabetes. Impaired fasting glucose (IFG) was considered in the presence of glucose levels between 100 and 126 mg/dL. NODAT was characterized as persistent diabetes occurring for more than 30 days after LT.17,18
Dyslipidemia was considered in the presence of high LDL and/or triglycerides and/or low HDL. Elevated LDL values were considered in the presence of levels higher than 100 mg/dL for diabetics and 130 mg/dL for non-diabetics. High triglycerides were considered as levels greater than 150 mg/dL. Accordingly, low HDL levels were assumed in the presence of values lower than 40 mg/dL for men and 50 mg/dL for women.16 Abnormal ferritin levels were considered in the presence of values greater than 300 ng/mL. Metabolic syndrome was diagnosed according to NCEP-ATP III criteria.16 Homeosta-sis model assessment (HOMA-IR)16 was calculated in all patients according to the formula: insulin (μU/mL) x glucose (mmol/L)/22.5 and IR was considered in the presence of HOMA-IR values higher than 3.017. HOMA-β was calculated as 20 x insulin (μU/mL)/glucose (mmol/ L) -3.5. β-cell dysfunction was evaluated by HOMA-β assessment and was considered in the presence of values lower than 167.19
After blood withdrawal, all patients were submitted to ultrasound (US) evaluation performed by a single radiologist (M.A.) with experience in the diagnosis of hepatic st-eatosis. The evaluation of steatosis was performed by conventional B-mode ultrasonography and was graded semiquantitatively as 1 and 2.20 The finding of steatosis by US was considered as evidence of NAFLD after LT.
All patients with NAFLD and abnormal liver enzymes were submitted to liver biopsy in order to stablish the diagnosis of NASH and histological findings were interpreted and scored according to established criteria.21
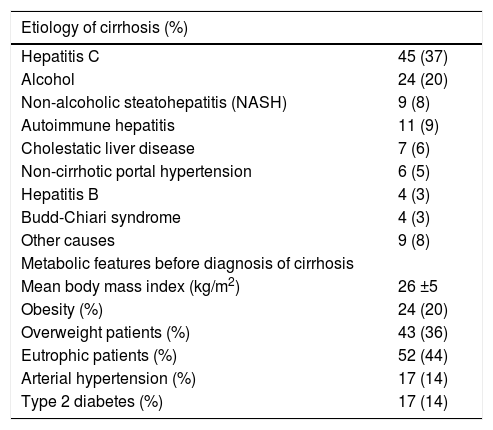
The database of LT and, when required, the clinical charts of the patients were also reviewed in order to search for demographic, anthropometric and other clinical and laboratory variables available before LT, including the presence of past history of AHT, type 2 diabetes and obesity prior to LT. Weight before the diagnosis of cirrhosis was considered for BMI assessment before LT. The indications for LT in those subjects were decompensated cirrhosis (n = 83) and HCC (n = 36). The clinical and laboratory parameters of those subjects before LT are depicted in table 1. Most had cirrhosis due to hepatitis C and alcohol and only 9 (8%) had NASH.
Clinical and laboratory features of the patients before liver transplantation (n = 119).
| Etiology of cirrhosis (%) | |
|---|---|
| Hepatitis C | 45 (37) |
| Alcohol | 24 (20) |
| Non-alcoholic steatohepatitis (NASH) | 9 (8) |
| Autoimmune hepatitis | 11 (9) |
| Cholestatic liver disease | 7 (6) |
| Non-cirrhotic portal hypertension | 6 (5) |
| Hepatitis B | 4 (3) |
| Budd-Chiari syndrome | 4 (3) |
| Other causes | 9 (8) |
| Metabolic features before diagnosis of cirrhosis | |
| Mean body mass index (kg/m2) | 26 ±5 |
| Obesity (%) | 24 (20) |
| Overweight patients (%) | 43 (36) |
| Eutrophic patients (%) | 52 (44) |
| Arterial hypertension (%) | 17 (14) |
| Type 2 diabetes (%) | 17 (14) |
Liver transplantation was performed in all patients with standard techniques using veno-venous bypass. All patients used triple-drug immunosuppression that consisted of tacrolimus, prednisone, azathioprine from 2001 to 2004 and mycophenolate mofetil (MMF) thereafter. Withdrawal of prednisone and azathioprine or MMF was attempted as per protocol in all patients after 6 and 12 months after LT, respectively. All biopsy-proven or suspected acute al-lograft rejection episodes were treated, according to severity, with an increase in tacrolimus dosages or less frequently with intravenous bolus of methylprednisolone.
This study has been approved by the Ethics Committee of the University Hospital of Federal University of Bahia, Brazil and followed the ethical guidelines of the Declaration of Helsinki.
Statistical AnalysisData were expressed in text and tables as mean and standard errors or or median and interquartile range for variables not normally distributed. Comparison of categorical variables was performed using χ2 or Fischer's exact test, when appropriate. Continuous variables were compared using T Student or Mann-Whitney tests as appropriate. Analysis of variance (ANOVA) or Kruskal-Wallys test were used to compare continuous variables between patient groups as appropriate. A p value less than 0.05 was considered significant. All statistical analysis was performed using IBM SPSS statistics version 21 (SPSS Inc., Chicago, IL, USA).
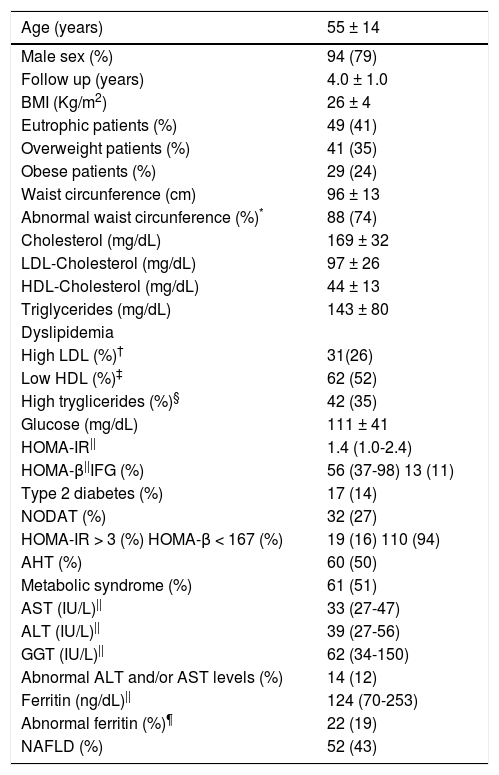
ResultsTwo hundred and ninety six patients were submitted to LT due to end-stage liver disease or HCC between December 2001 and August 2014. One-hundred twenty-three subjects died before enrollment and 54 were either excluded or were not willing to take part in the study. One hundred and nineteen patients (94 males, mean age 55 ± 14 years) were prospectively evaluated. Clinical and laboratory features of those patients at the time of evaluation after a mean follow-up period of 4.0 ± 1.0 years after LT are disclosed in table 2. The immunosuppressive regimens employed by those subjects were tacrolimus (n = 75), tacrolimus and MMF (n = 34), tacrolimus and prednisone (n = 3), cyclosporine (n = 5), MMF (n = 2). Only ten patients had acute cellular rejection treated with increasing doses of tacrolimus, with the exception of one subject that required three intravenous bolus of methyl-prednisolone more than two years before entry in the study.
Clinical and laboratory features of patients after liver transplantation at the time of evaluation (n = 119).
| Age (years) | 55 ± 14 |
|---|---|
| Male sex (%) | 94 (79) |
| Follow up (years) | 4.0 ± 1.0 |
| BMI (Kg/m2) | 26 ± 4 |
| Eutrophic patients (%) | 49 (41) |
| Overweight patients (%) | 41 (35) |
| Obese patients (%) | 29 (24) |
| Waist circunference (cm) | 96 ± 13 |
| Abnormal waist circunference (%)* | 88 (74) |
| Cholesterol (mg/dL) | 169 ± 32 |
| LDL-Cholesterol (mg/dL) | 97 ± 26 |
| HDL-Cholesterol (mg/dL) | 44 ± 13 |
| Triglycerides (mg/dL) | 143 ± 80 |
| Dyslipidemia | |
| High LDL (%)† | 31(26) |
| Low HDL (%)‡ | 62 (52) |
| High tryglicerides (%)§ | 42 (35) |
| Glucose (mg/dL) | 111 ± 41 |
| HOMA-IR|| | 1.4 (1.0-2.4) |
| HOMA-β||IFG (%) | 56 (37-98) 13 (11) |
| Type 2 diabetes (%) | 17 (14) |
| NODAT (%) | 32 (27) |
| HOMA-IR > 3 (%) HOMA-β < 167 (%) | 19 (16) 110 (94) |
| AHT (%) | 60 (50) |
| Metabolic syndrome (%) | 61 (51) |
| AST (IU/L)|| | 33 (27-47) |
| ALT (IU/L)|| | 39 (27-56) |
| GGT (IU/L)|| | 62 (34-150) |
| Abnormal ALT and/or AST levels (%) | 14 (12) |
| Ferritin (ng/dL)|| | 124 (70-253) |
| Abnormal ferritin (%)¶ | 22 (19) |
| NAFLD (%) | 52 (43) |
Most of the patients had full-blown MS or at least one of its major determinants (Table 2). Comparison of the frequencies of diabetes, AHT and either overweight or obesity before and after LT revealed a significant increase in the frequency of AHT (14% vs. 50% after LT, p < 0.001) and type 2 diabetes or NODAT (14% vs. 41% after LT, p < 0.001), without major difference in the distribution of overweight and obesity (56% vs. 59% after LT, p = NS) (Tables 1 and 2). Of those diabetic patients, 32 (27%) had NODAT and the remaining had persistent type 2 diabetes after LT, as none had disease remission after surgery (Table 2). Patients with type 2 diabetes and/or NODAT were under treatment at time of the evaluation with met-formin (n = 18), insulin (n = 17), glibenclamide (n = 7), metformin and insulin (n = 4), dapagliflozin (n = 2), lina-gliptin (n = 1). In addition, IFG was observed in 11% of the patients. The median HOMA-IR and HOMA-β levels in all patients were 1.4 [1.0-2.4] and 56 [37-98], respectively (Table 2). As expected, higher levels of HOMA-IR were observed in patients with type 2 diabetes and/or NODAT when compared to their non-diabetic counterparts (2.3 [1.1-3.4] vs. 1.2 [0.9 -1.8] in non-diabetic patients, p = 0.001). Insulin resistance, defined as HOMA-IR > 3, was seen in only 15 (30%) subjects with NODAT and/or diabetes and 4 (6%) non-diabetic subjects (p = 0.001) (Table 3). Likewise, lower HOMA-β levels were disclosed in subjects with NODAT and/or diabetes when compared to their non-diabetic counterparts (40 [24-55] vs. 78 [50-110] in non-diabetic subjects, p < 0.01). HOMA-β values were under the normal range in 110 (94%) patients, 48 (98%) subjects with NODAT and/or diabetes and 64 (91%) non-diabetic subjects (p = NS) (Table 3).
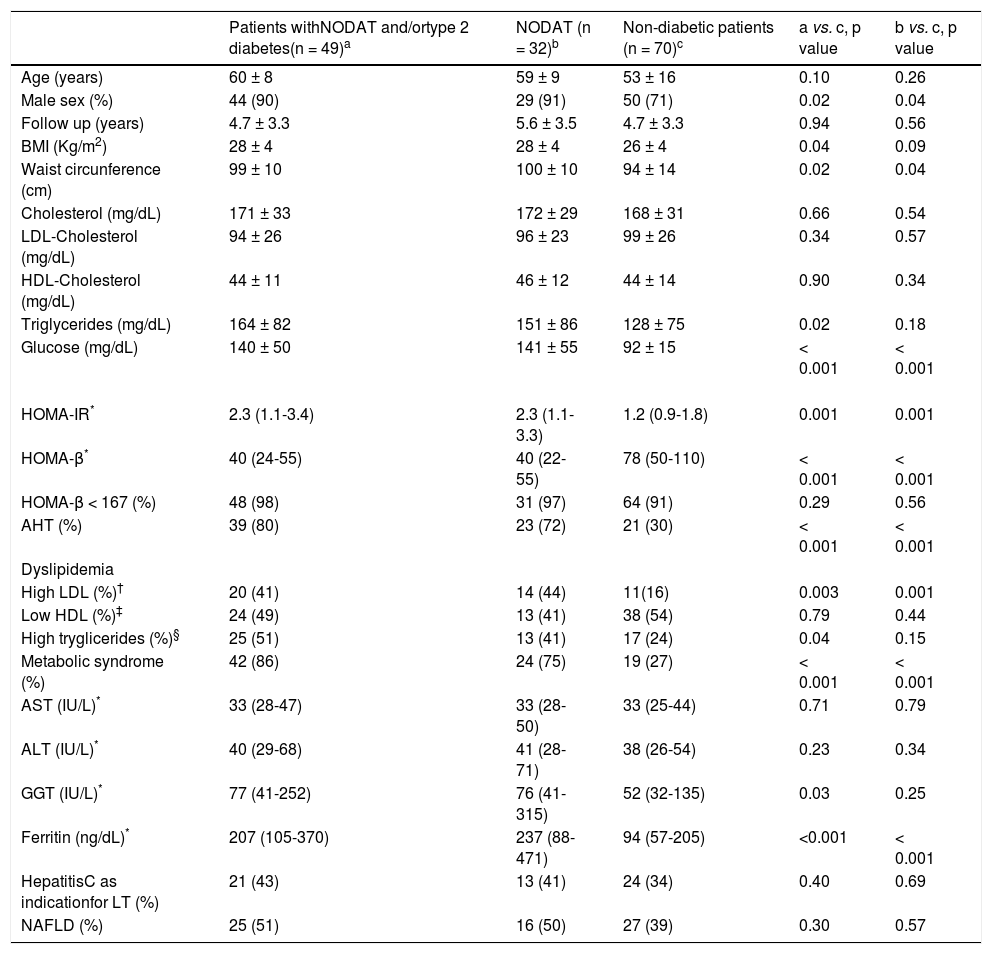
Clinical and laboratory features of patients after liver transplantation according to the presence of diabetes
| Patients withNODAT and/ortype 2 diabetes(n = 49)a | NODAT (n = 32)b | Non-diabetic patients (n = 70)c | a vs. c, p value | b vs. c, p value | |
|---|---|---|---|---|---|
| Age (years) | 60 ± 8 | 59 ± 9 | 53 ± 16 | 0.10 | 0.26 |
| Male sex (%) | 44 (90) | 29 (91) | 50 (71) | 0.02 | 0.04 |
| Follow up (years) | 4.7 ± 3.3 | 5.6 ± 3.5 | 4.7 ± 3.3 | 0.94 | 0.56 |
| BMI (Kg/m2) | 28 ± 4 | 28 ± 4 | 26 ± 4 | 0.04 | 0.09 |
| Waist circunference (cm) | 99 ± 10 | 100 ± 10 | 94 ± 14 | 0.02 | 0.04 |
| Cholesterol (mg/dL) | 171 ± 33 | 172 ± 29 | 168 ± 31 | 0.66 | 0.54 |
| LDL-Cholesterol (mg/dL) | 94 ± 26 | 96 ± 23 | 99 ± 26 | 0.34 | 0.57 |
| HDL-Cholesterol (mg/dL) | 44 ± 11 | 46 ± 12 | 44 ± 14 | 0.90 | 0.34 |
| Triglycerides (mg/dL) | 164 ± 82 | 151 ± 86 | 128 ± 75 | 0.02 | 0.18 |
| Glucose (mg/dL) | 140 ± 50 | 141 ± 55 | 92 ± 15 | < 0.001 | < 0.001 |
| HOMA-IR* | 2.3 (1.1-3.4) | 2.3 (1.1-3.3) | 1.2 (0.9-1.8) | 0.001 | 0.001 |
| HOMA-β* | 40 (24-55) | 40 (22-55) | 78 (50-110) | < 0.001 | < 0.001 |
| HOMA-β < 167 (%) | 48 (98) | 31 (97) | 64 (91) | 0.29 | 0.56 |
| AHT (%) | 39 (80) | 23 (72) | 21 (30) | < 0.001 | < 0.001 |
| Dyslipidemia | |||||
| High LDL (%)† | 20 (41) | 14 (44) | 11(16) | 0.003 | 0.001 |
| Low HDL (%)‡ | 24 (49) | 13 (41) | 38 (54) | 0.79 | 0.44 |
| High tryglicerides (%)§ | 25 (51) | 13 (41) | 17 (24) | 0.04 | 0.15 |
| Metabolic syndrome (%) | 42 (86) | 24 (75) | 19 (27) | < 0.001 | < 0.001 |
| AST (IU/L)* | 33 (28-47) | 33 (28-50) | 33 (25-44) | 0.71 | 0.79 |
| ALT (IU/L)* | 40 (29-68) | 41 (28-71) | 38 (26-54) | 0.23 | 0.34 |
| GGT (IU/L)* | 77 (41-252) | 76 (41-315) | 52 (32-135) | 0.03 | 0.25 |
| Ferritin (ng/dL)* | 207 (105-370) | 237 (88-471) | 94 (57-205) | <0.001 | < 0.001 |
| HepatitisC as indicationfor LT (%) | 21 (43) | 13 (41) | 24 (34) | 0.40 | 0.69 |
| NAFLD (%) | 25 (51) | 16 (50) | 27 (39) | 0.30 | 0.57 |
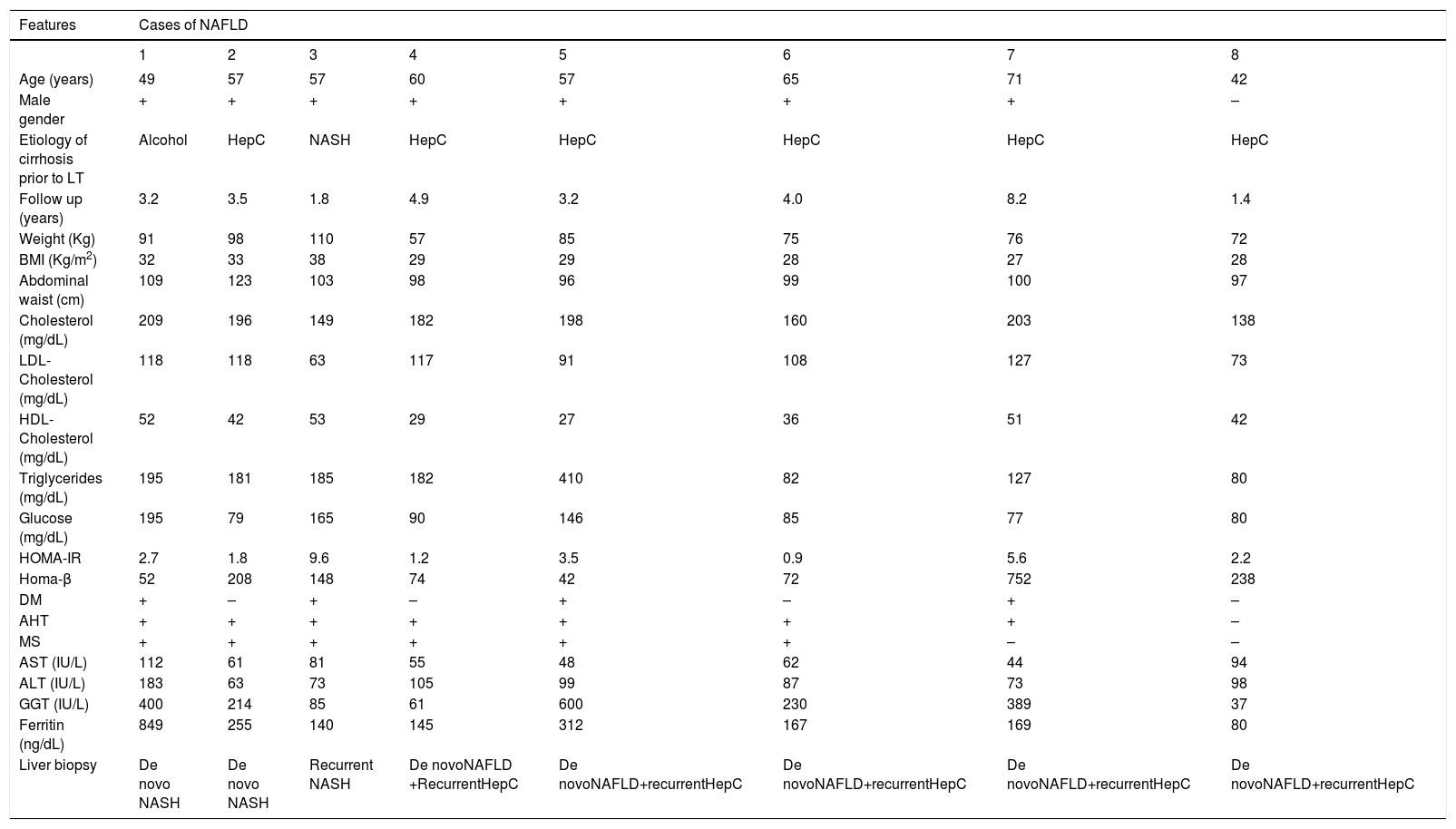
Abnormal ALT and/or AST values were seen in 14 patients. All were submitted to liver biopsy. Ultrasound evaluation disclosed steatosis in 52 (43%) patients. Recurrent NAFLD by imaging was seen in 5 out of 9 (56%) patients who received transplants for NASH. De novo NAFLD was observed in 47 (43%) subjects with other etiologies for cirrhosis prior to LT. In those subjects with NAFLD by imaging, ALT and/or AST were abnormal in 11 (9%). Liver biopsy revealed either simple steatosis (n = 5) or NASH (n = 3) in 8 (72%) of those patients. Recurrent hepatitis C (n = 2) and de novo autoimmune hepatitis (n = 1) were diagnosed in those patients without histological evidence of NAFLD. All of those patients with simple steatosis also had genotype 1 hepatitis C virus. Graft dysfunction in those patients was attributed to recurrent viral disease based on virological and histological findings. Three patients had NASH. Two of them, each previously transplanted for hepatitis C and alcoholic cirrhosis, had de novo NASH. The other patient had recurrent NASH due to the presence of HCC associated with NASH before LT. The clinical and laboratory features of those eight patients with either recurrent or de novo NAFLD are depicted in table 4. All had either obesity or MS. Only three subjects had IR assessed by HOMA-IR levels and all but one patient had normal ferritin values.
Clinical, laboratory and histological features of NAFLD patients after LT with abnormal liver enzymes.
| Features | Cases of NAFLD | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Age (years) | 49 | 57 | 57 | 60 | 57 | 65 | 71 | 42 |
| Male gender | + | + | + | + | + | + | + | – |
| Etiology of cirrhosis prior to LT | Alcohol | HepC | NASH | HepC | HepC | HepC | HepC | HepC |
| Follow up (years) | 3.2 | 3.5 | 1.8 | 4.9 | 3.2 | 4.0 | 8.2 | 1.4 |
| Weight (Kg) | 91 | 98 | 110 | 57 | 85 | 75 | 76 | 72 |
| BMI (Kg/m2) | 32 | 33 | 38 | 29 | 29 | 28 | 27 | 28 |
| Abdominal waist (cm) | 109 | 123 | 103 | 98 | 96 | 99 | 100 | 97 |
| Cholesterol (mg/dL) | 209 | 196 | 149 | 182 | 198 | 160 | 203 | 138 |
| LDL-Cholesterol (mg/dL) | 118 | 118 | 63 | 117 | 91 | 108 | 127 | 73 |
| HDL-Cholesterol (mg/dL) | 52 | 42 | 53 | 29 | 27 | 36 | 51 | 42 |
| Triglycerides (mg/dL) | 195 | 181 | 185 | 182 | 410 | 82 | 127 | 80 |
| Glucose (mg/dL) | 195 | 79 | 165 | 90 | 146 | 85 | 77 | 80 |
| HOMA-IR | 2.7 | 1.8 | 9.6 | 1.2 | 3.5 | 0.9 | 5.6 | 2.2 |
| Homa-β | 52 | 208 | 148 | 74 | 42 | 72 | 752 | 238 |
| DM | + | – | + | – | + | – | + | – |
| AHT | + | + | + | + | + | + | + | – |
| MS | + | + | + | + | + | + | – | – |
| AST (IU/L) | 112 | 61 | 81 | 55 | 48 | 62 | 44 | 94 |
| ALT (IU/L) | 183 | 63 | 73 | 105 | 99 | 87 | 73 | 98 |
| GGT (IU/L) | 400 | 214 | 85 | 61 | 600 | 230 | 389 | 37 |
| Ferritin (ng/dL) | 849 | 255 | 140 | 145 | 312 | 167 | 169 | 80 |
| Liver biopsy | De novo NASH | De novo NASH | Recurrent NASH | De novoNAFLD +RecurrentHepC | De novoNAFLD+recurrentHepC | De novoNAFLD+recurrentHepC | De novoNAFLD+recurrentHepC | De novoNAFLD+recurrentHepC |
Hep C: Hepatitis C. NASH: Non-alcoholic steatohepatitis. NAFLD: Non-alcoholic fatty liver disease.
Comparison of clinical and laboratory variables according to the presence of type 2 diabetes or NODAT after LT revealed that diabetic patients were more commonly males, had more frequently AHT, MS and IR. They also had significantly higher BMI, waist circumference, HOMA-IR, tryglicerides, LDL-cholesterol, GGT and ferritin values, as well as lower HOMA-β levels, when compared to their non-diabetic counterparts. Similar findings were also disclosed in those patients with NODAT when compared to non-diabetic subjects, with the exception of BMI, tryglicerides and GGT levels that were not significantly different between the aforementioned groups of patients (Table 3).
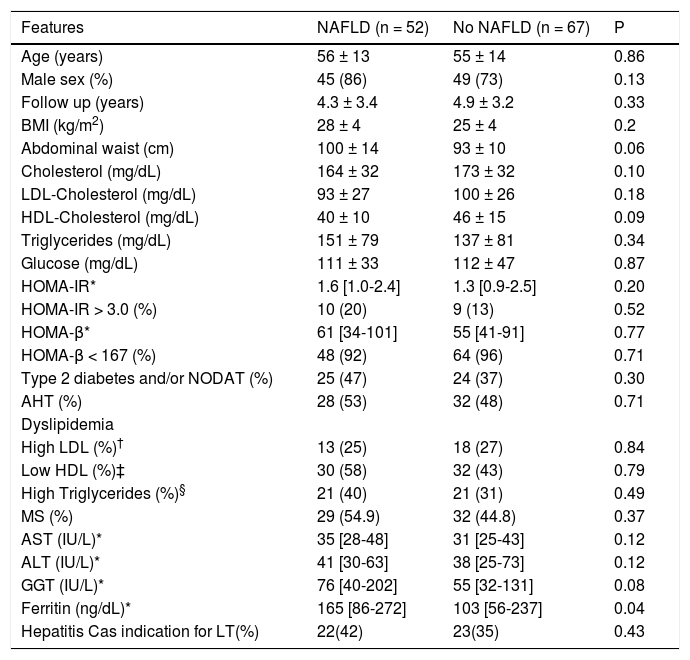
No difference in the frequencies of clinical and laboratory parameters, including either type 2 diabetes or NODAT, in patients according to the presence of NAFLD after LT (Table 5), with the exception of ferritin levels, which were significantly higher in those subjects with NAFLD (165 [86-272] vs. 103 [56-237] in patients without NAFLD, (p = 0.04) (Table 4). Similar frequencies of MS were observed in patients with and without NAFLD (Table 4). In addition, there was no difference in the frequencies of IR in those patients with and without MS (16% vs. 4% of the subjects without MS, p = NS).
Clinical and laboratory features of patients after liver transplantation according to the presence of NAFLD.
| Features | NAFLD (n = 52) | No NAFLD (n = 67) | P |
|---|---|---|---|
| Age (years) | 56 ± 13 | 55 ± 14 | 0.86 |
| Male sex (%) | 45 (86) | 49 (73) | 0.13 |
| Follow up (years) | 4.3 ± 3.4 | 4.9 ± 3.2 | 0.33 |
| BMI (kg/m2) | 28 ± 4 | 25 ± 4 | 0.2 |
| Abdominal waist (cm) | 100 ± 14 | 93 ± 10 | 0.06 |
| Cholesterol (mg/dL) | 164 ± 32 | 173 ± 32 | 0.10 |
| LDL-Cholesterol (mg/dL) | 93 ± 27 | 100 ± 26 | 0.18 |
| HDL-Cholesterol (mg/dL) | 40 ± 10 | 46 ± 15 | 0.09 |
| Triglycerides (mg/dL) | 151 ± 79 | 137 ± 81 | 0.34 |
| Glucose (mg/dL) | 111 ± 33 | 112 ± 47 | 0.87 |
| HOMA-IR* | 1.6 [1.0-2.4] | 1.3 [0.9-2.5] | 0.20 |
| HOMA-IR > 3.0 (%) | 10 (20) | 9 (13) | 0.52 |
| HOMA-β* | 61 [34-101] | 55 [41-91] | 0.77 |
| HOMA-β < 167 (%) | 48 (92) | 64 (96) | 0.71 |
| Type 2 diabetes and/or NODAT (%) | 25 (47) | 24 (37) | 0.30 |
| AHT (%) | 28 (53) | 32 (48) | 0.71 |
| Dyslipidemia | |||
| High LDL (%)† | 13 (25) | 18 (27) | 0.84 |
| Low HDL (%)‡ | 30 (58) | 32 (43) | 0.79 |
| High Triglycerides (%)§ | 21 (40) | 21 (31) | 0.49 |
| MS (%) | 29 (54.9) | 32 (44.8) | 0.37 |
| AST (IU/L)* | 35 [28-48] | 31 [25-43] | 0.12 |
| ALT (IU/L)* | 41 [30-63] | 38 [25-73] | 0.12 |
| GGT (IU/L)* | 76 [40-202] | 55 [32-131] | 0.08 |
| Ferritin (ng/dL)* | 165 [86-272] | 103 [56-237] | 0.04 |
| Hepatitis Cas indication for LT(%) | 22(42) | 23(35) | 0.43 |
NAFLD: Non-alcoholic fatty liver disease. * Median and interquartile ranges are depicted for skewed variables; † Considered in the presence of levels higher than 100 mg/dL for diabetics and 130 mg/dL for non-diabetics. ‡ Considered in the presence of levels lower than 40 mg/dL for men and 50 mg/dL for women. § Considered in the presence of levels higher than 150 mg/dL.
New onset diabetes after liver transplantation was observed in 32 (27%) subjects in the present study after a median follow-up of 4 ± 1 years. All 17 patients with type 2 diabetes before LT remained with type 2 diabetes thereafter and the overall prevalence of either type 2 diabetes or NODAT after LT was 41%. Those findings are in accordance with previous studies that disclosed NODAT in 11%-37% of the patients after LT.2,22,23 In those reports, risk factors for NODAT were male gender,2,23 age,22 BMI,22,23 hepatitis C infection,2,22,23 IFG prior to LT23 and tacrolimus-based immunosuppression.22,23 In the present investigation, NODAT was also correlated with male gender and high BMI values, but not with increasing age or hepatitis C infection. In addition, AHT, MS, IR and β-cell dysfunction were more commonly observed in patients with either type 2 diabetes and/or NODAT. HOMA-IR and HOMA-β are common tools employed, respectively, for the quantitative assessment of IR and β-cell dysfuc-tion, particularly in non-diabetic subjects. In this regard, HOMA-IR values higher than 3.0 and HOMA-β less than 167 have been considered, respectively, as indicative of the presence of IR and β-cell dysfunction in different popula-tions.5,19 Higher HOMA-IR and lower HOMA-β were disclosed in the patients with type 2 diabetes and/or NODAT, but the frequency of IR in the whole cohort of patients was lower than expected, as only 19 (16%) of them had HOMA-IR values > 3. β-cell dysfunction, on the other hand, was overrepresented in patients with or without diabetes, as only seven subjects had HOMA-β values ≥ 167. This may reflect the employment of tacrolimus-based im-munossupression in all of the patients. Tacrolimus is a cal-cineurin inhibitor more diabetogenic when compared to cyclosporine.2 It can induce NODAT through pancreatic beta cell toxicity, leading to impaired insulin production and/or secretion.24 These findings altogether point out to the fact that the NODAT frequently reported in patients submitted to LT may be more drug-induced and less frequently associated to IR and MS.
This study has also disclosed NAFLD in 43 % of the patients after LT. Using this strategy, recurrent and de novo NAFLD were observed in 56% and 43% of the patients, respectively. Those findings are in accordance with previous reports that described recurrent and de novo NAFLD after LT in 25%-70%9 and 18%-33%3,4,11,14 of the patients, respectively.
Occurrence of de novo NAFLD was associated in some of those reports with several pre-transplantation variables including alcoholic cirrhosis3,4 donor graft steato-sis3 and high recipient BMI before LT.4,13 It was also correlated with other metabolic complications frequently encountered after LT in some3,4,11,13 but not all9,14 reports, such as weight gain and obesity3,11,13 either type 2 diabetes or NODAT,3,4 hyperlipidemia,3,4 and AHT.3 None of those clinical and laboratory features were associated with de novo NAFLD in the present study, despite the high prevalence of some of those metabolic disturbances observed in this cohort of patients, since one third to one half of them had either type 2 diabetes and/or NODAT or dyslipidemia and half of them had AHT. Metabolic syndrome is reported to occur in 33%-58% of liver transplant recipients3,25 and was also observed in 51% of the patients in the present study. It is characterized by the presence of those aforementioned metabolic disorders, particularly type 2 diabetes, dyslipidemia, AHT, and central obesity.16 Insulin resistance is the main trigger for MS and the driving force behind the development of type 2 diabetes and NAFLD.5,6 It is also associated with progression of fibro-sis in NASH. Few studies have addressed the presence of IR in those subjects.25 In the present study, IR was seen in only 16% of the subjects after LT and was not associated either with MS or NAFLD, suggesting that IR was not a major trigger for the development of those metabolic derangements in this cohort. Other authors have associated recurrent NAFLD with MS, AHT and either type 2 diabetes or NODAT after LT.14 In those reports, recurrent but not de novo NAFLD evolved more frequently to progressive fibrosis and cirrhosis.14 In this regard, fibrosis was reported to occur in 18% of those patients with recurrent NAFLD but only in 2% to 4% of those subjects with de novo NAFLD.3,4,14 In the present study, histological evaluation was undertaken only in those patients with NAFLD and abnormal liver enzymes. NAFLD was not confirmed by liver biopsy in three out of 11 subjects. The other subjects had either de novo (n = 7) or recurrent (n = 1) NAFLD, including those three subjects with de novo (n = 2) and recurrent (n = 1) NASH. All of the three patients submitted to liver biopsy with recurrent (n = 1) or de novo (n = 2) NASH had no fibrosis. It is important to emphasize that NAFLD was assessed by the findings of st-eatosis by US in the present study and that liver biopsy was performed only in those patients with biochemical abnormalities. All other studies evaluating the frequency of recurrent or de novo NAFLD reviewed liver biopsy samples obtained per protocol3,4,13,14 or for evaluation of graft dysfunction.11,12 This may account for the higher frequency of NASH with or without fibrosis seen in previous studies in comparison to the present study, probably due to selection bias, inasmuch more severe patients would be more prone to undergo liver biopsy. It is also important to acknowledge that US is less accurate when compared to histology for the diagnosis of NAFLD. One recent meta-analysis has described a pooled sensitivity and specificity for US of 85% and 94% for the detection of moderate to severe steatosis.20 It is also important to stress that accuracy also tend to be lower with lower degrees of fat accumulation.26 It is however improbable that severe cases could have been missed using this US-based strategy for the diagnosis of NAFLD after LT. Previous studies have also shown a negative correlation of normal ALT values and NASH on liver biopsy after LT. Moreover, even in non-transplanted subjects with normal ALT, normal HOMA-IR values (seen in 89% of our the NAFLD patients) were also associated with a non-aggressive histology.27 Most of the patients in the present study had normal ferritin levels, but their values were significantly higher in those subjects with type 2 diabetes, NODAT and NAFLD. Ferritin is a recognized marker of inflammation and has been associated in several studies in non-transplant patients28 with the presence of NAFLD, as well as with a more aggressive course of the disease with development of NASH with fibrosis or cir-rhosis.29
This study has some limitations, particularly due to the number of patients enrolled and the shortened follow-up to assess graft survival. It has to be acknowledged that NASH is a diagnosis that can only be made or ruled-out by histology and that biopsy was performed in this study only in patients with biochemical signs of graft dysfunction. On the contrary, the present investigation is, to our knowledge, the first report to assess HOMA-IR and HOMA-β levels in subjects with NAFLD after LT, to link NODAT more closely to calcineurin β-cell toxicity rather than to IR and to suggest that de novo NAFLD after LT may be benign in the absence of IR.
In summary, NODAT, recurrent or de novo NAFLD were frequently observed in the postoperative course of LT. Metabolic syndrome and its determinants were often present without laboratory signs of IR, probably due to complications of immunossupression. The impact of NAFLD in graft dysfunction was not shown to be significant, probably accounting for the increased survival associated with transplantation for NASH, recently reported.15
AcknowledgementsThe authors would like to thank Dr. Andrea Ribeiro Cavalcanti, Dr. Magid Abbud, Dr. Luiz Antonio Rod-rigues de Freitas and Ms. Izabel Cristina de Queiroz Batista, Portuguese Hospital of Salvador, Bahia, Brazil, for collecting data for the study and Dr. Cláudia Alves Couto, University of Minas Gerais, Brazil for revising the manuscript.
Conflict of InterestNone.
Financial SupportNone.