We are at the cusp of significant new alternatives for the treatment of chronic hepatitis C. Among more than 100 drugs in development, some are ready to be approved and in the market as soon as next year. The protease inhibitors Telaprevir and Boceprevir, will change the SOC treatment to triple therapy, (combined with peg IFN and RBV), with duration of treatment guided by rapid virological response. In this article we present the data supporting the approval of Telaprevir and Boceprevir, and information on polymerase inhibitors and IFN free proof of concept trials. Finally we discuss which patients should wait for DAA based therapies and which should be considered for peg IFN/RBV now.
In the next 5 years our patients can expect higher response rates and truncated duration of therapy. They can expect drug cocktails or combos but for the next years these novel drugs will still require peg IFN and RBV. Also, a new era of resistance as a barrier to therapy will require sub typing and more viral monitoring. Overall, improved outcomes will come at the expense of more adverse events and increased costs of treatment.
We are at the cusp of significant new alternatives for the treatment of chronic hepatitis C. More than 100 new drugs, most oral, are at different stages of development, and some may be available as soon as mid 2011.
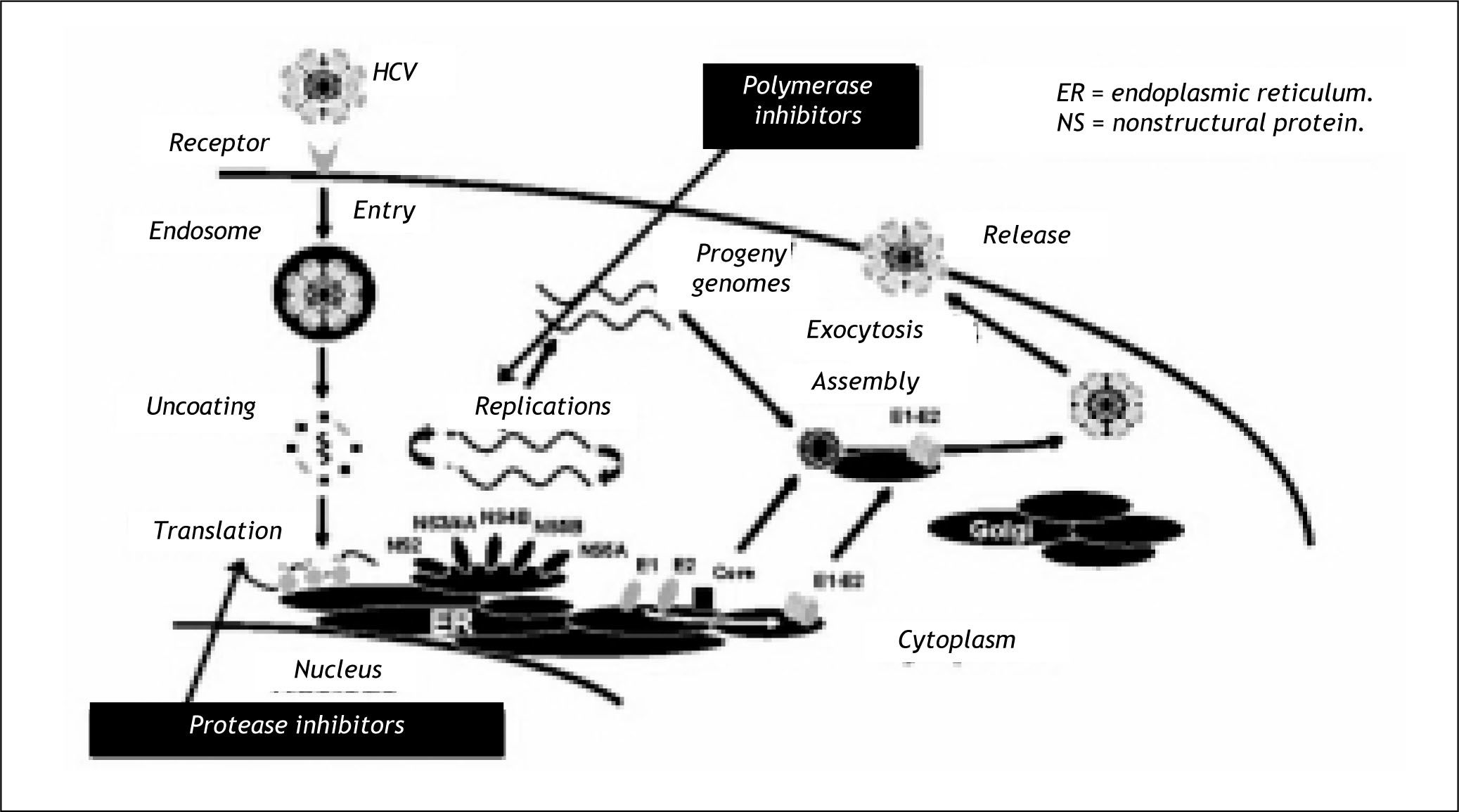
Figure 1 shows some of the most promising sites of intervention for these new therapies; including Glycosylation inhibitors, Oligonucleotides and siR-NA, Cyclophillin inhibitors and finally the protease and polymerase inhibitors, the drugs more advanced in development.
So, in view of these evolving therapies how we should approach the CHC patient in 2010?
The first consideration should be the likelihood of response to the available therapies of Peg-IFN and RBV. The important variables are genotype, viral load, ethnicity and/or race and lately the presence of IL28B genotype.1 Latino patients have a lower efficacy with available therapies.2
The second consideration is the likelihood of disease progression in hepatic histology. If the disease is mild patients can wait for better therapies.
The final consideration is the likelihood of tolerating treatment with peg-IFN and RBV.
We will present the available information about the more advanced drugs in development in order to try to select those patients that could or should be offered therapy now, and which patients should wait for additional therapies.
The most advanced drugs in development are the protease and polymerase inhibitors. The protease inhibitors interfere with the protease crucial for the translation of the hepatitis C virus and the polyme-rase inhibitors act further along the chain interfering with the viral replication (Figure 1).3
Among the protease inhibitors in development the more advanced are : Vertex / Tibotec: Telaprevir (VX-950); Schering-Plough: Boceprevir; Tibotec: TMC435350; Boehringer Ingelheim: BI 201335; Intermune / Roche: ITMN-191 = R7227; and Merck: MK-7009. The protease inhibitors were the first Directly Acting Antiviral drugs developed, and paved the way for the understanding of important aspects of viral resistance in addition to demonstrating im-proved efficacy rates.
First the results from the Telaprevir and Boce-previr clinical trials have shown that therapy with peg IFN and RBV plus a protease inhibitor will achieve efficacy rates in excess of 70% for genotype 1 naive patients.
Second, the early studies with Telaprevir were instrumental to demonstrate that therapy with peg IFN and RBV were required to prevent the emergence of mutations to Telaprevir.4
The emergence of resistance was also associated to Telaprevir through concentrations in the presence of peg IFN.5
Lately, this information has become more relevant with reports about the significant number of naive patients that harbor drug resistant mutations at NS3 protease or NS5B polymerase to a panel of 27 drugs (gen 1a-21.5%; genlb 44.4% and gen 3a 41.8%).6
Let us examine the most advanced protease inhibitors in development; Telaprevir and Boceprevir. Both drugs have completed enrollment in their phase 3 studies and plan for new drug application to the regulatory agencies this year, with possible approval for 2011.
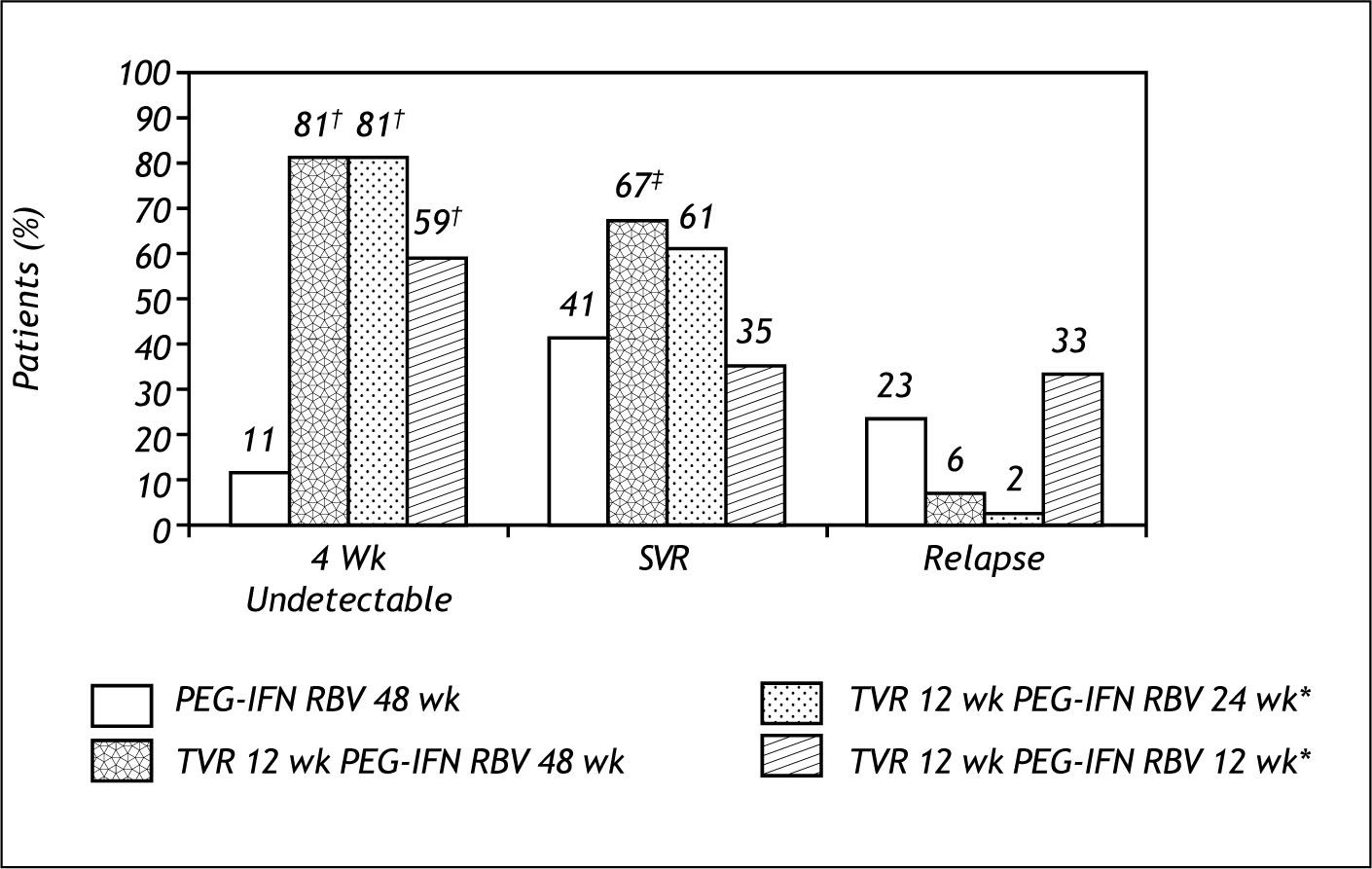
Telaprevir clinical trials: NaïveProve 1Patients in the total duration arms of 24 and 48 weeks had similar SVR rates. Patients that only received 12 weeks of total duration had lower SVR, similar to SOC, at the expense of high relapse rates. This study also confirmed that adverse events of rash, nausea and diarrhea were more frequent in the Telaprevir arms (Figure 2).7
Prove 1 also shown that virologic breakthrough occurred in 7 % of cases due to the development of resistance to Telaprevir, and that it occurred more frequently during the first 4 weeks of treatment and in patients without rapid virological response (ND at week 4).It was also demonstrated that genetic barriers to resistance vary among sub types; gen1a 9% and gen1b 2% (Figure 3).
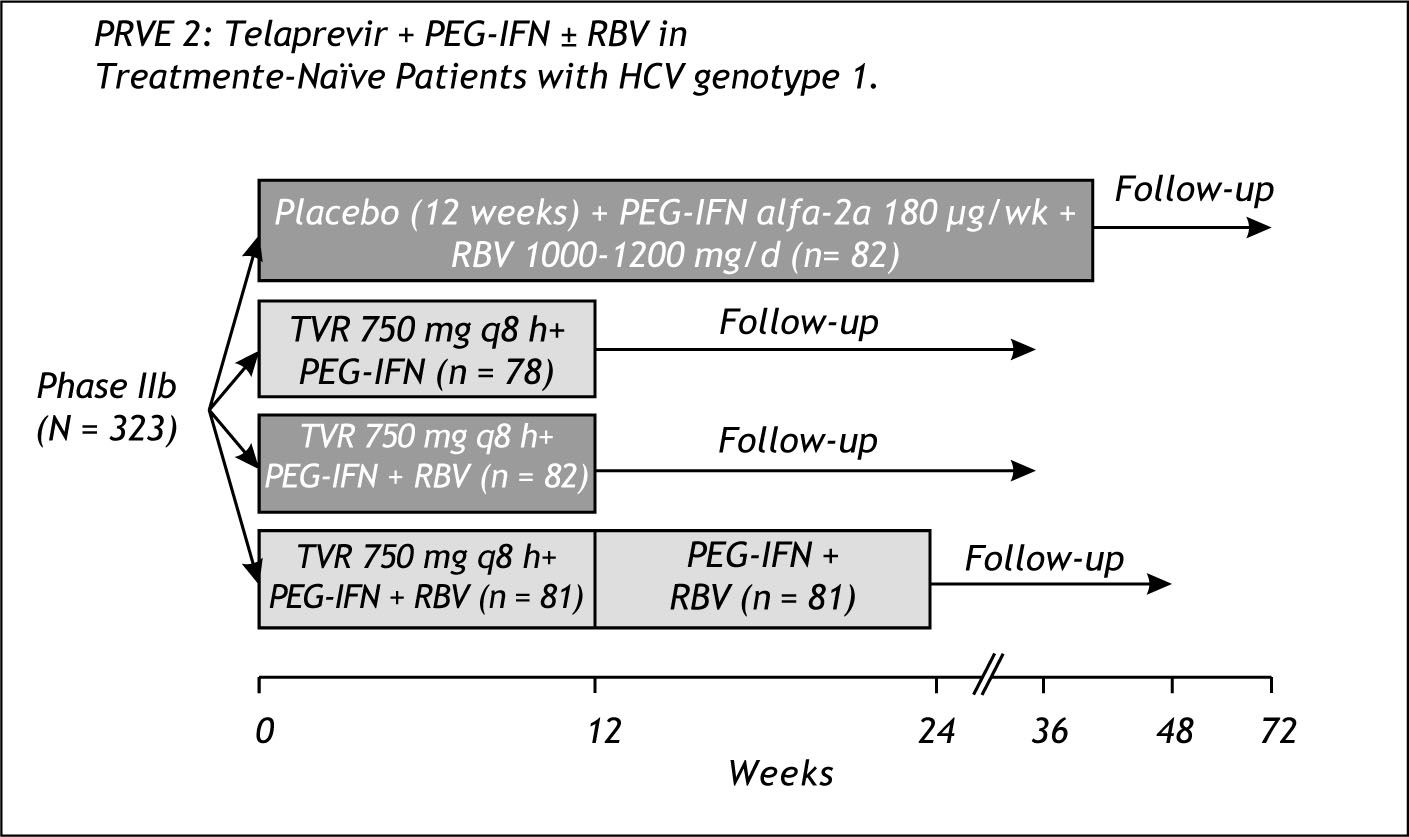
Prove 2Prove 2 was conducted in Europe and had an arm without RBV with a total duration of only 12 weeks-Figure 4.
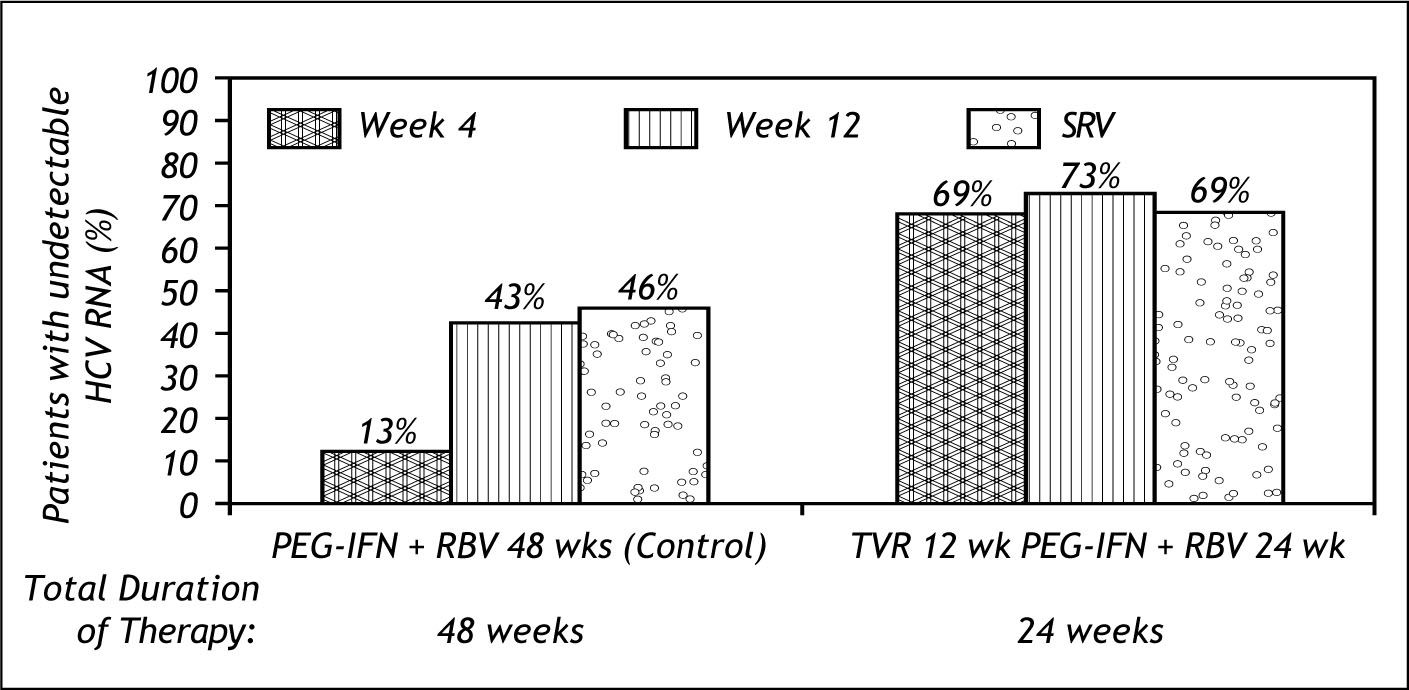
The results of prove 2 are shown in figure 5. 69% of patients achieved SVR with only 24 weeks total duration and only 12 weeks of Te-laprevir.8
This study showed that RBV is needed to prevent relapse; 48% in the RBV free arm relapsed. It also showed that RBV may have an impact on resistance, as resistant mutations were low in the triple therapy arms (3%) and high in the RBV free arm (24%).9
The results of the Prove 1 and 2 studies demonstrated the improved efficacy of the Telaprevir based 24 weeks total duration therapy in difficult to treat patients; patients older than 50 years old, with high viral load, males and patients with bridging cirrho-sis.10
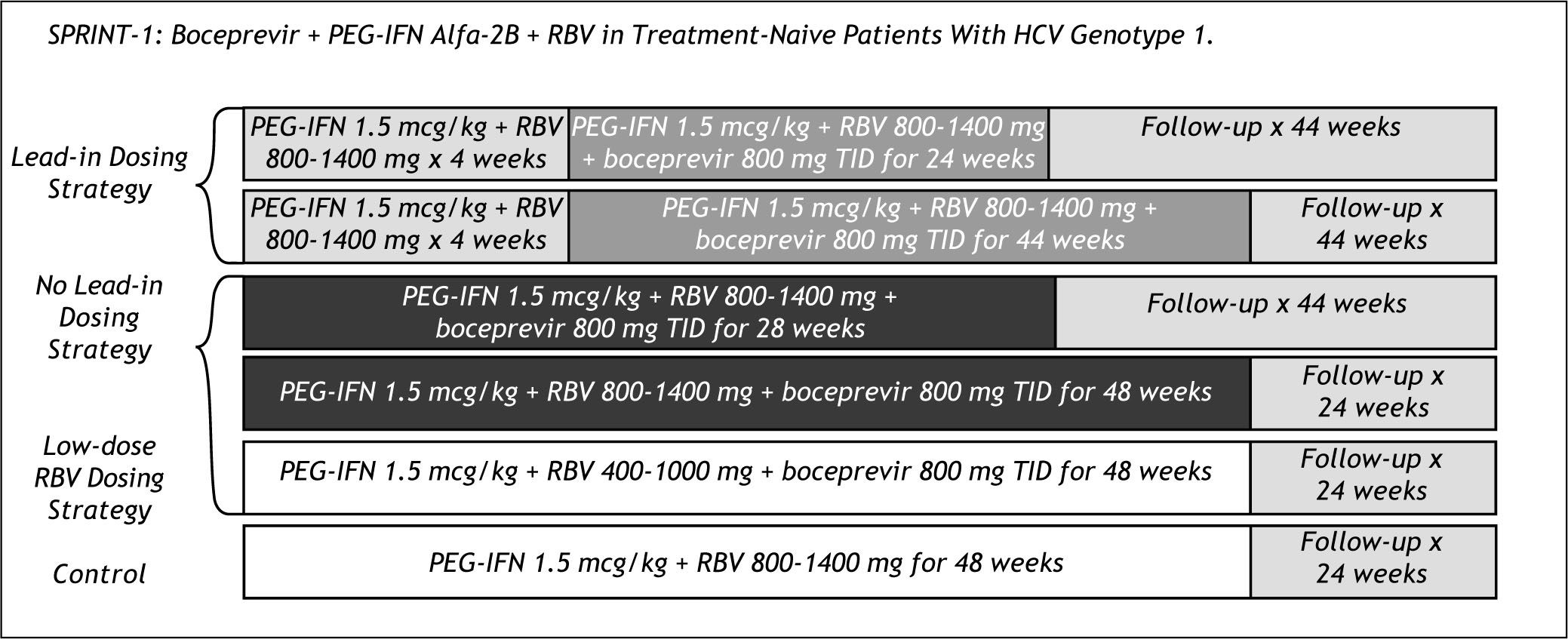
Boceprevir clinical trials: Naïve patientsSprint 1Figure 6 shows the rather complex design of the Sprint 1 trial. The study had arms with and without a Lead in of 4 weeks of peg IFN and RBV before starting Boceprevir dosing and an arm with lower doses of RBV.
Sprint 1 resulted in 56% SVR with 28 weeks total duration and the lead in of 4 weeks, compared to 67% with 48 weeks duration and 75% with 48 weeks therapy and 4 weeks of lead in. Patients in the low RBV dose arm had lower than SOC efficacy; 36 vs. 50 % SVR.
Boceprevir based therapy had significant adverse events, especially anemia in more than 55% of patients, and dysgeusia in more than 30%. In the case of anemia, Epoietin alfa was used in more than 45% of all patients.
Clinical trials: Experienced patientsRetreatment of non responders to prior peg IFN based therapy, results in very poor efficacy rates, not higher than 16%.11,12 The response rates for re-lapsers is superior with 38% efficacy on re treat-ment.11
Both Telaprevir and Boceprevir trials have conducted trials in experienced patients. The results of Telaprevir based therapy has been reported on the Prove 3 study and the phase 3 registration trial in experienced patients, Realize, has been completely enrolled.
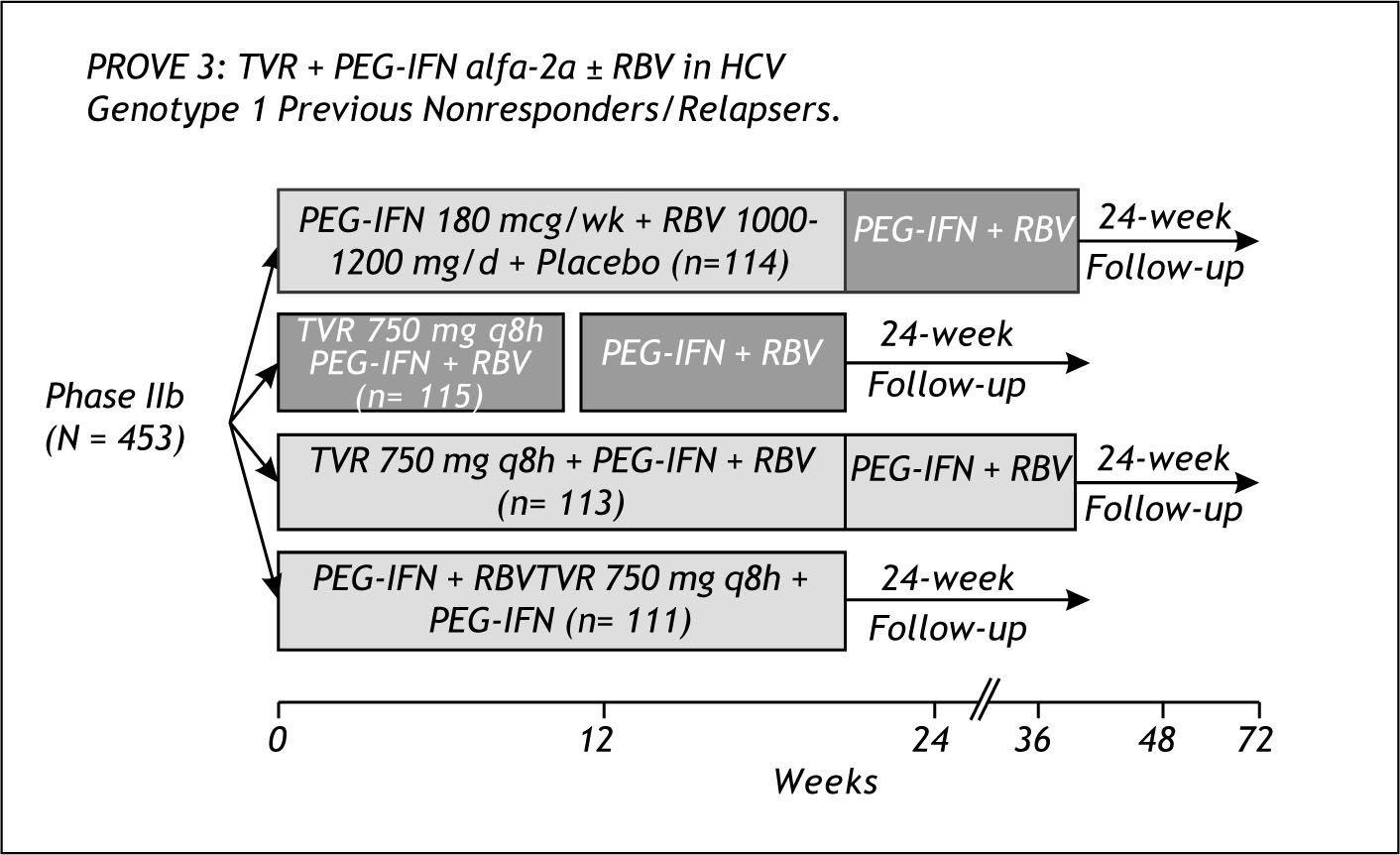
Prove 3Figure 7 shows the design of Prove 3. Both non responders and relapsers to prior peg IFN /RBV therapy, were included in this study. The study included 2 arms with total duration of 24 weeks, one arm without RBV and 12 weeks Telaprevir dosing and the other with 8 weeks of Telaprevir plus peg IFN and RBV. The 2 arms with total duration of 48 weeks were the control group and an arm of Telaprevir plus peg IFN/RBV for 24 weeks and 24 additional weeks of peg/RBV.
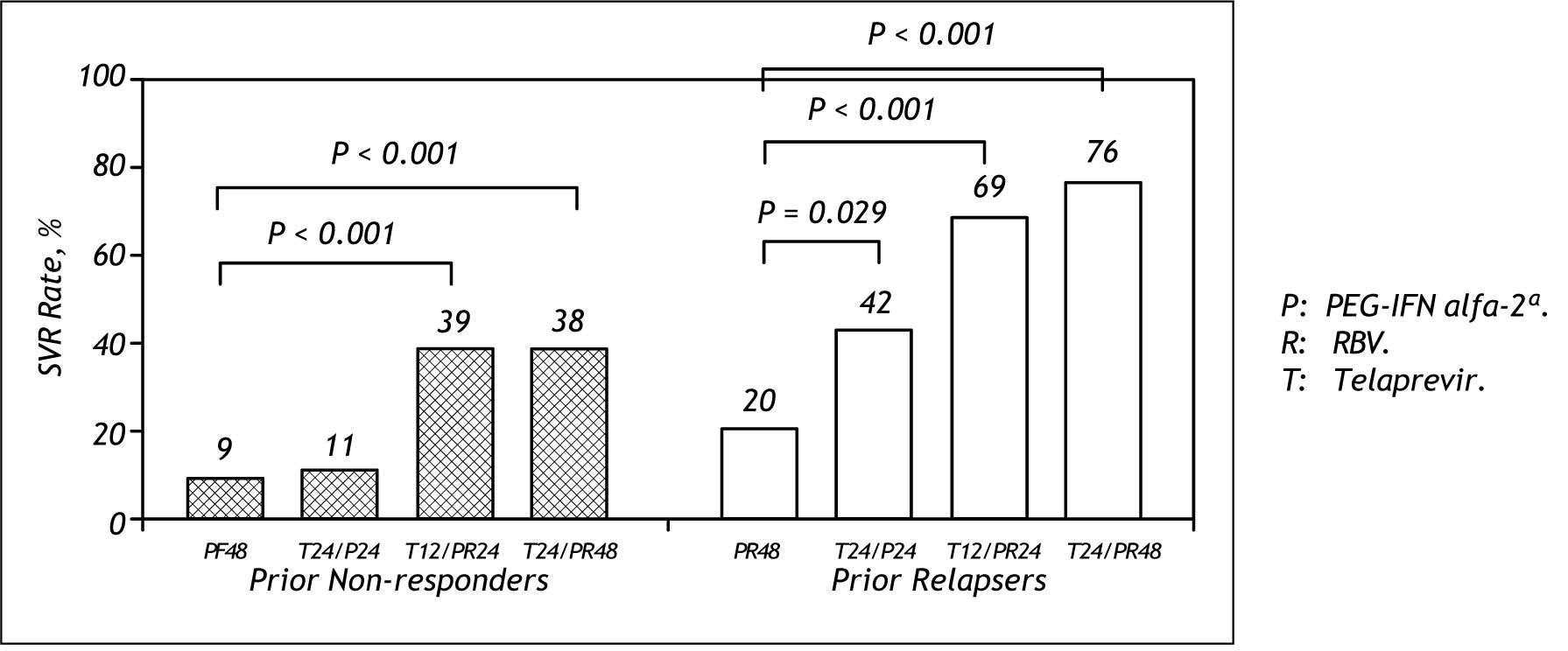
The results from Prove 3 are shown in figure 8. There was no difference in SVR between the Tela-previr based arm of 24 weeks or 48 weeks in prior non responders. (39 vs. 38%) For relapsers, 69% achieved SVR with 24 weeks Telaprevir based therapy compared to 76% with 48 weeks duration.13
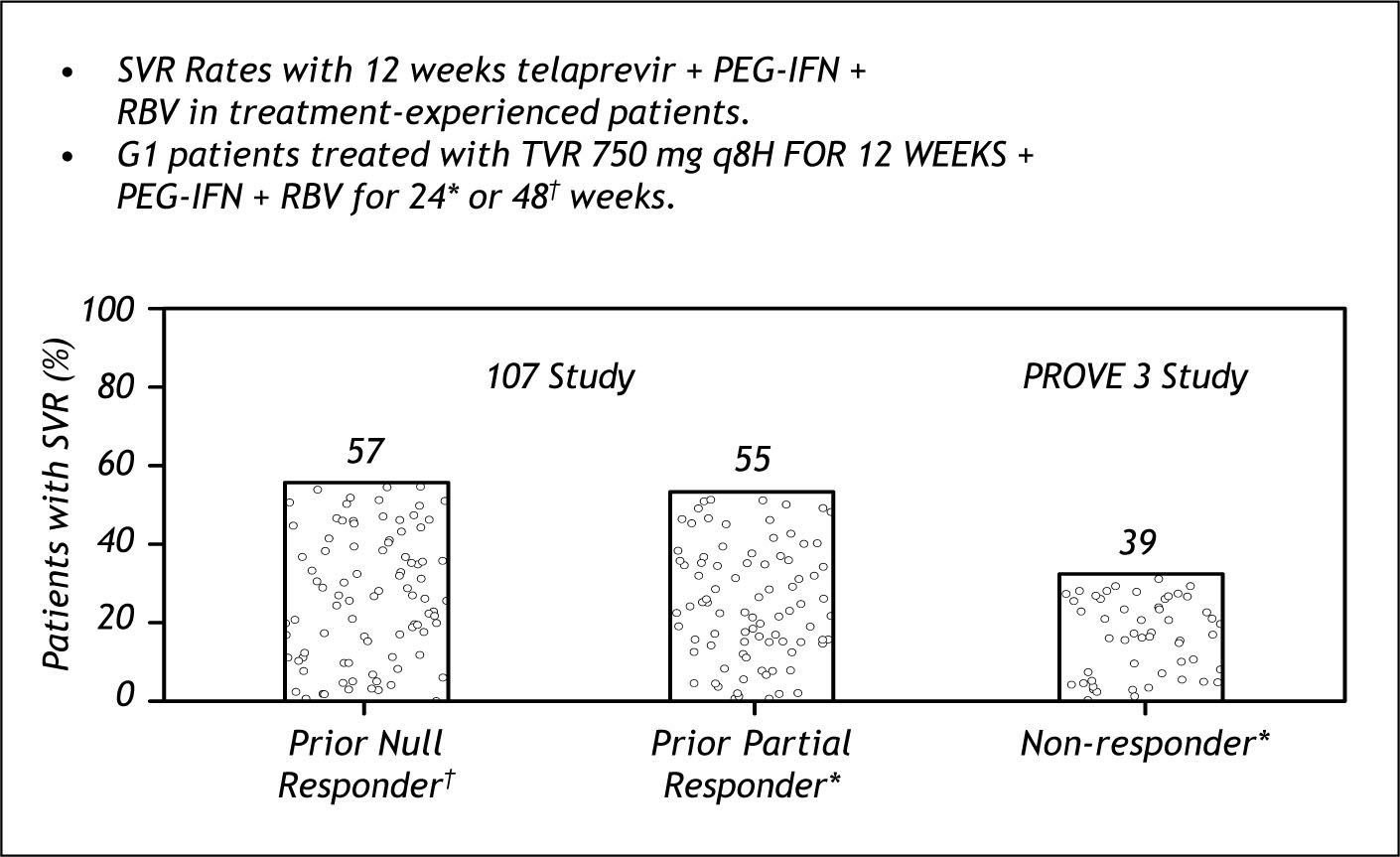
Further studies have been performed in non res-ponders with Telaprevir based therapy(Study 107).This was a roll over study of patients that participated in prove 1 or 2 and were randomized to placebo arms and were non responders.
Figure 9, shows the efficacy of therapy on Null Responders, prior Partial Responders and compares the results to the findings of Prove 3 that did not di-fferentiate among those. The results of 107 show the same SVR rates among the non responders catego-ries.14
Polymerase inhibitorsThe NS5B polymerase inhibitors are either nu-cleoside analogs or non nucleoside inhibitors. The nucleoside analogs are active site inhibitors that require conversion to active triphosphate. The nu-cleosides affect a highly conserved region of the genome, and have a high genetic barrier to resistance. These inhibitors are active against genotype 2 and 3.
The non nucleosides perform stereotactic inhibition away from the catalytic site of a not highly conserved region, and as a consequence have a lower barrier to resistance.
- •
The following polymerase inhibitors are in advanced development:Pharmasset / Roche: R7128; Idenix: IDX-375; Pfizer: PF00868554; Genelabs / Novartis: GL60067; Anadys: ANA598; Vertex / ViroChem VCH-222.
None of these drugs will be available for the next 3 years.Phase 2 trials are underway and phase 3 trials are expected to start next year.
IFN free treatmentsA hope for the future is to achieve IFN and RBV free treatments against HCV that could be efficacious and allow to bypass the side effects of present therapies. Althoug we are too far away of that goal, the proff of concept of that possibility has been reported.
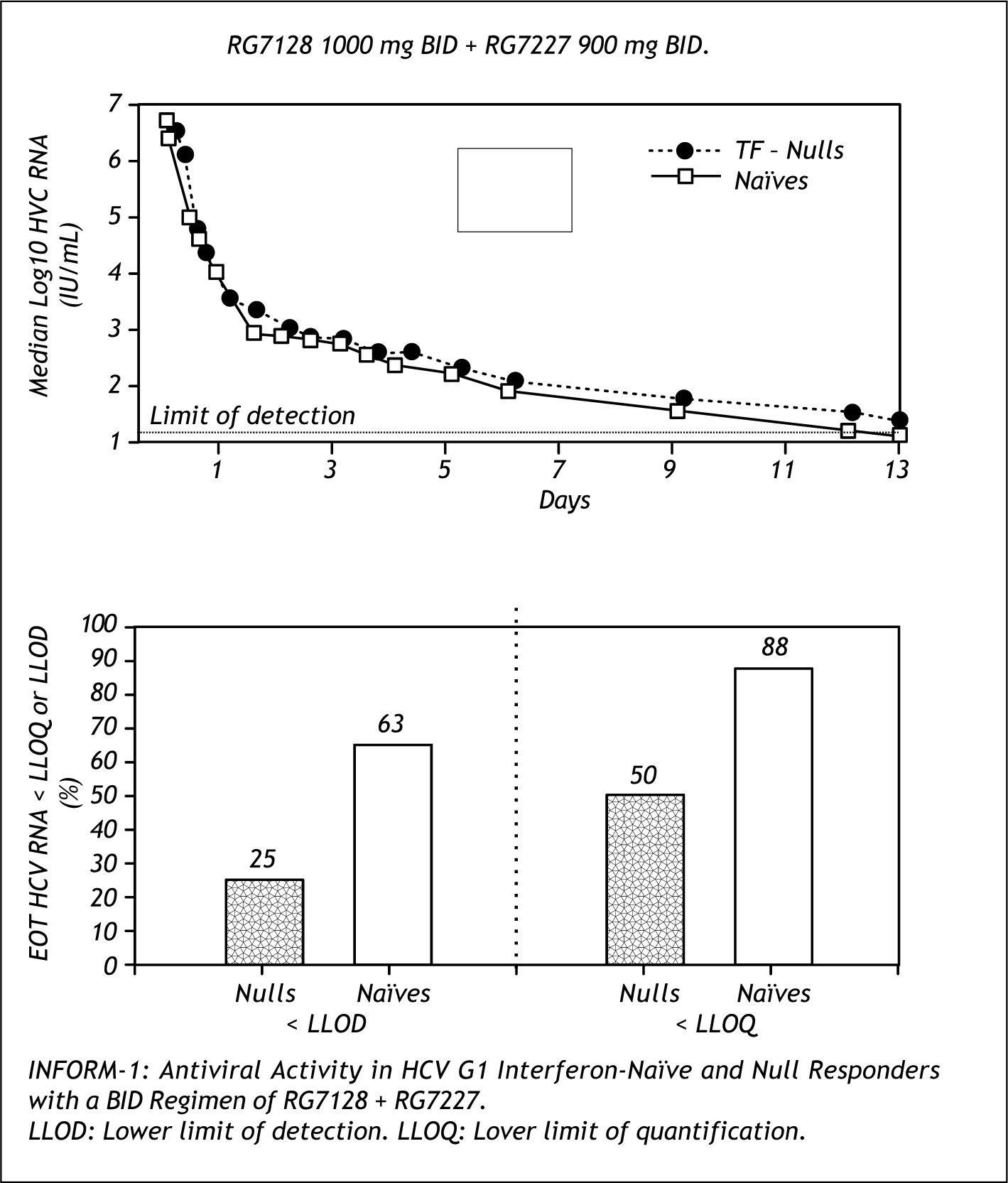
Figure 10 shows the results of the INFORM trial, an early study of the combination of RG7128 (poly-merase inhibitor) and RG7227(protease inhibitor) in naive and non responders dosed for 13 days. This combination was able to achieve dramatic decreases of HCV PCR, with 88% of naives and 50% of non responders reaching lower level of quantification.14
Further combination studies are planned with these two drugs.
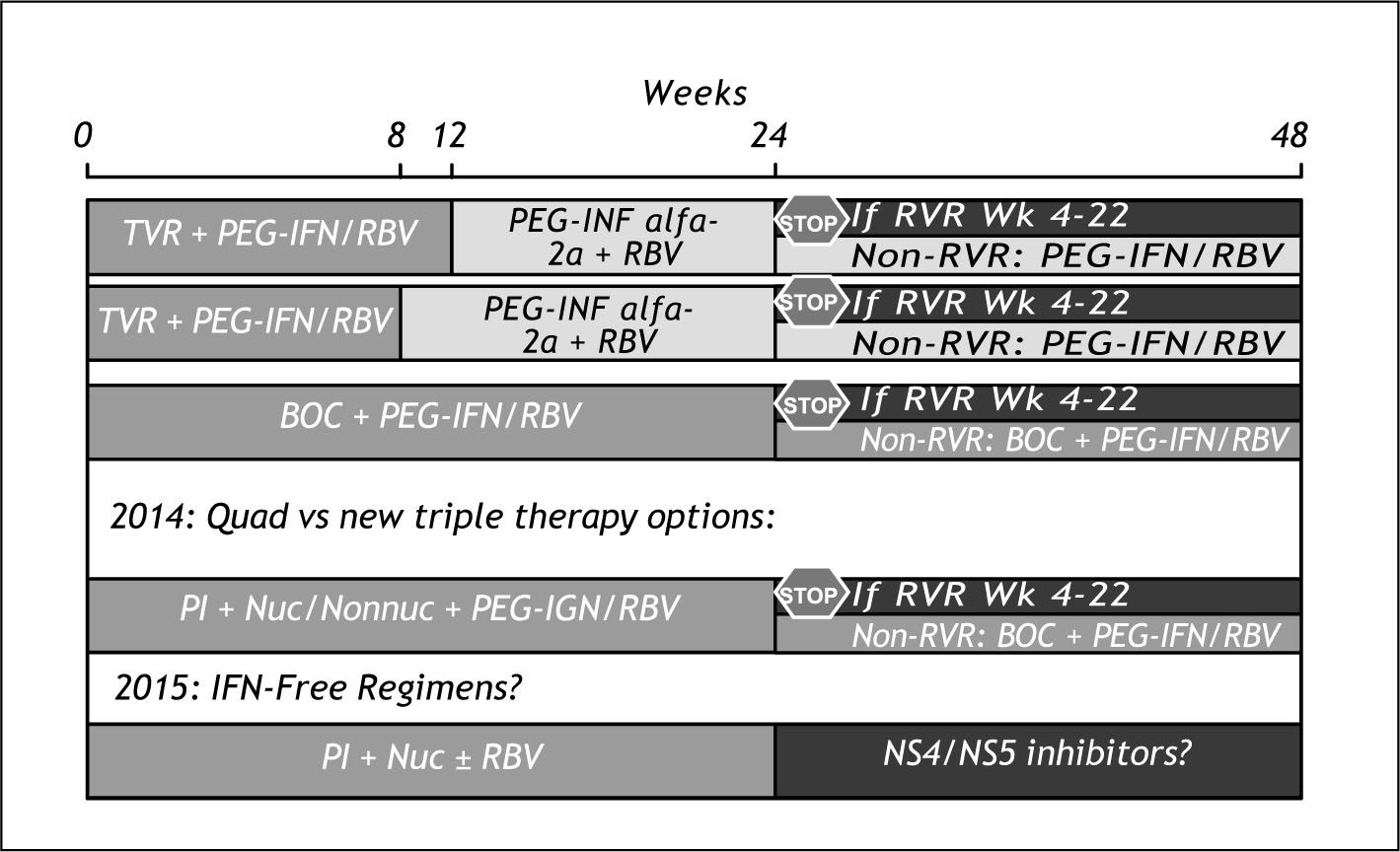
Evolution of therapyFigure 11 shows our thoughts about what may happen in the next years. For 2011, we expect to have both Telaprevir and Boceprevir approved by the regu-latory agencies and entering the market. The SOC will be then triple therapy, with a duration of treatment decided by rapid virological response or not. The duration of Telaprevir dosing will be no more than 12 weeks while for Boceprevir dosing will be 24 weeks or more for a definite advantage for telaprevir. Telaprevir will have also the advantage of much lower risk of anemia and need of use of erythropoietin.
For 2013-2014 we expect to have 4 drug combinations and most of the patients achieving SVR with no more than 24 weeks of therapy.
By 2015 we should have IFN and RBV free regimens available. We think that will be able to get rid of IFN before than RBV because we suspect RBV was our first DAA drug.
To treat now or to wait?
So, what would be our recommendations to our patients?
- •
Gen1,4-naive without advanced liver disease-wait for DAA’s. The efficacy rates for white Latino genotype 1 patients is around 34%.2 Much improved rates are seen with telaprevir and Boce-previr based therapies.
- •
Gen1,4-naive with significant disease may benefit of therapy now with Peg/RBV. Still these patients could be treated with triple therapy if do not respond.
- •
Gen 2/3 –naive-treat with Peg/RBV. The protease inhibitors that will be approved next year are not effective against these genotypes. The polymerase inhibitors will not be approved for years. SVR rates of around 65% in Latinos.2
- •
Relapser without adequate prior therapy-re treat with Peg/RBV or wait till 2011.
- •
Relapsers with adequate prior therapy – wait for DAA’s.
- •
Non responders-wait for DAA’s.
In the next years the management of CHC therapy will change dramatically with new rules to be followed during therapy.
Strict follow up for development of mutations and breakthrough is going to be the rule. This will result in:
- •
More frequent HCV RNA tests.
- •
Discontinuation at week 4 for all patients without at least a 1 log decrease.
- •
Discontinuation of all patients without cEVR.
- •
Discontinuation of all patients with any breakthrough.
- •
Not only genotype, but phenotype and sensitivity tests will be available and used to decide and monitor therapy as soon as 2011.
What can our patients expect in 2011 to 2015?
Our patients can expect higher response rates and truncated duration of therapy with rapid virolo-gic response. They can expect drug cocktails or combos with different oral drugs. However, for the next years these novel drugs will still require peg IFN and RBV. Also, a new era of resistance as a barrier to therapy starts, that will require subtyping and more viral monitoring. In addition, improved outcomes will come with added adverse events and increased costs of treatment.














![Efficacy of the therapy. McHutchison JG, et al, (AALSD Abstract 66) Hepatology, 2009; 50(S4): 334A-335A. Vertex Pharmaceuticals. [news release]. Cambridge, MA. October 28 2008.http://investors.vrtx.com/releaseDetail.cfm?releaseid=419250. Efficacy of the therapy. McHutchison JG, et al, (AALSD Abstract 66) Hepatology, 2009; 50(S4): 334A-335A. Vertex Pharmaceuticals. [news release]. Cambridge, MA. October 28 2008.http://investors.vrtx.com/releaseDetail.cfm?releaseid=419250.](https://static.elsevier.es/multimedia/16652681/00000009000000S1/v1_201906221034/S1665268119317375/v1_201906221034/en/main.assets/thumbnail/gr9.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)






