Background and rationale for the study. The purpose of this study was to assess the technical and clinical outcomes of transjugular intrahepatic portosystemic shunt (TIPS) reduction for the management of TIPS-induced acute liver decompensation. Between August 2000 and November 2013, 347 patients underwent a TIPS procedure in the authors’ institution; 21/347 (6%) developed post-TIPS acute liver decompensation which was managed using a percutaneous shunt reduction technique. Patient demographics, laboratory tests before and after initial TIPS and TIPS reduction, procedural data and clinical follow-up data were analysed.
Results. Twenty-one patients (mean age 63 years) who underwent an initial TIPS procedure for variceal bleeding (n = 7; 33%) or refractory ascites (n = 14; 67%) successfully underwent shunt reduction ten days (3-34 days) after the initial TIPS procedure. The portosystemic pressure gradient (PSPG) increased from 8 (3-17) mmHg before reduction to 12 (7-23) mmHg after shunt reduction. Survival at one and six months follow-up was 15 (71%) and 11 patients (52%), respectively. The international normalised ratio (INR) (1.7 vs. 1.5; p = 0.044) was significantly different after TIPS reduction in the non-survival group compared to the survival group. In conclusion, TIPS reduction for the management of TIPS-induced acute liver decompensation is technically feasible and is associated with a one and six-month mortality rate of 29% and 48%, respectively. Higher post-TIPS-reduction INR values may be associated with higher risk of early mortality.
The creation of a transjugular intrahepatic portosystemic shunt (TIPS) may be associated with several complications, both local procedural, including puncture-related, and more general shunt-related, including hepatic encephalopathy and acute liver decompensation.1
Acute liver decompensation in patients with pre-existing liver cirrhosis is defined as an acute deterioration of liver function and subsequently the functioning of other end organs over a period of weeks following a precipitating event, either indirect (variceal haemorrhage, sepsis) or direct (drug-induced, liver resection, hepatotoxic factor and TIPS placement), in a patient with previously well-compensated or reasonably well-compensated chronic liver disease.2–4
When transplantation is not an option, TIPS occlusion or reduction may ameliorate the degree of liver insufficiency.5,6 The rationale of TIPS reduction is to narrow the shunt flow lumen while preserving its patency, thereby increasing portal blood perfusion of the liver. The technique of TIPS reduction is the same as described for the interventional radiological management of TIPS-induced hepatic encephalopathy.7–10 The purpose of this study was to characterise the technical, haemodynamic, laboratory and clinical outcomes of TIPS reduction in the treatment of TIPS-induced acute liver decompensation.
Material and MethodsStudy designAn electronic search in the institution’s interventional radiology database was performed to identify patients who underwent an initial TIPS procedure between August 2000 and November 2013 and, owing to the development of TIPS-induced acute liver decompensation, were later referred for an interventional procedure for TIPS reduction. TIPS-induced acute liver decompensation was defined as liver decompensation associated with the development of jaundice (≥ 50% increase in bilirubin levels compared to pre-TIPS) and coagulopathy (≥ 50% increase in International Normalised Ratio (INR) compared to pre-TIPS), complicated by ascites or encephalopathy or both ≤ 4 weeks after a TIPS procedure. This definition excludes liver decompensation elicited by infection or gastrointestinal bleeding. Clinical success was defined as improvement of patient’s general and biochemical status, allowing discharge from the intensive care unit.
Pre and post-interventional clinical, laboratory and radiological data for these patients were collected retrospectively using patients’ electronic medical and radiological hospital records. Approval by the local Ethics Committee was obtained for retrospective data analysis.
Interventional radiological technique for initial TIPS and TIPS reduction procedure:
In all 21 patients, the initial portosystemic shunt was created as previously described.11 Briefly, percutaneous access to the right internal jugular vein was obtained under general anaesthesia. Pressure measurements were performed in the inferior vena cava using a pigtail catheter and in the right hepatic vein using an occlusion balloon catheter (wedged hepatic vein pressure measurement) to calculate the PSPG. Carbon dioxide (CO2) wedged portography was performed to guide the puncture from the right hepatic vein into the right proximal portal vein using a coaxial Rösch-Uchida puncture set (Cook Medical, Bloomington, IN, USA). Following predilatation of the shunt tract (Wanda 5 mm diameter angioplasty balloon catheter, Boston Scientific, Natick, MA, USA), an expanded-polytetraethylene (ePTFE)-covered Nitinol stent graft (Viatorr, W. L. Gore & Associates, Flagstaff, AZ, USA) was inserted, with a nominal diameter of 10 mm and a length covering the intrahepatic parenchymal tract as far as the inferior vena cava. Afterwards, the stent graft was postdilated using a 10 mm diameter angioplasty balloon (Wanda; Boston Scientific, Natick, MA, USA) in the case of variceal bleeding and using an 8 mm angioplasty balloon (Wanda; Boston Scientific) in the case of refractory ascites or hepatic hydrothorax. Finally, pressure measurements were performed in the inferior vena cava and in the portal vein main branch, respectively.
The TIPS reduction procedure was performed using the parallel technique.9,10 Briefly, depending on the patient’s general condition, the percutaneous TIPS reduction procedure was performed under either general or local anaesthesia. Percutaneous venous access was obtained to the right jugular and right common femoral vein, followed by catheterisation of the TIPS tract from both the jugular and femoral approach. The PSPG was calculated based on pressure measurements in the proximal inferior vena cava and in the portal vein main branch. Iodised contrast portography was performed to evaluate TIPS Stent graft patency. Shunt reduction was achieved by placing a stainless steel balloon-expandable 6 mm diameter and 17 mm long stent mounted on an 0.035 inch catheter system (Express Vascular, Boston Scientific, Natick, MA,USA), which was deployed in the middle third of the initial TIPS tract, in parallel with an ePTFE-covered stent graft (Viatorr, W.L. Gore & Associates) with a nominal diameter of 10 mm and the same length as the initial stent graft. Afterwards, completion portography and PSPG measurements were performed. In the event of reopacification of any variceal collaterals immediately after shunt reduction, these collaterals were preventively coil-embolised using 0.035 inch stainless steel coils (MR eye coils, Cook Medical, Bloomington, IN, USA).
Statistical analysisThe Mann-WWhitney U test was used to compare survivors and non-survivors on continuous variables. All tests were two-sided. A 5% significance level was assumed for all tests. All analyses were performed using SAS software, version 9.4 of the SAS system for Windows (SAS Institute Inc., Cary, NC, USA).
Results21/347 patients (6%), with a median age of 63 years (range 47-84 years) including 11 male patients (53%) who had undergone a TIPS procedure between August 2000 and January 2013 developed TIPS-induced acute liver decompensation. Pre-interventional duplex ultrasound revealed a fully patent hepatic artery, portal vein and stented TIPS tract in all patients.
The underlying liver diseases were alcoholic cirrhosis (n = 12; 57%); non-alcoholic steatohepatitis (n = 3; 14%); alpha-1-antitrypsin deficiency (n = 1; 5%); hepatitis C (n = 1; 5%); chronic auto-immune cirrhosis (n = 1; 5%); cryptogenic cirrhosis (n = 1; 5%); glycogenosis (n = 1; 5%) and auto-immune hepatitis (n = 1; 5%). Indications for TIPS placement were variceal bleeding (n = 6; 29%), hepatic hydrothorax (n = 1; 5%), ascites (n = 12; 57%) or a combination of ascites and hepatic hydrothorax (n = 2; 10%). The laboratory values for the 21 patients prior to the initial TIPS procedure are summarised in table 1.
Laboratory values before initial TIPS placement.
| Variable | Statistic | Patient study population |
|---|---|---|
| ALT (U/l) | N | 20 |
| Mean | 25.3 | |
| Std | 9.65 | |
| Median | 23.5 | |
| IQR | (20.5; 32.0) | |
| Range | (8.0; 49.0) | |
| Bilirubin (mg/dL) | N | 20 |
| Mean | 1.8 | |
| Std | 1.03 | |
| Median | 1.6 | |
| IQR | (1.1; 2.4) | |
| Range | (0.5; 4.2) | |
| INR | N | 21 |
| Mean | 1.3 | |
| Std | 0.18 | |
| Median | 1.3 | |
| IQR | (1.2; 1.4) | |
| Range | (1.0; 1.6) | |
| Creatinin (mg/dL) | N | 21 |
| Mean | 1.1 | |
| Std | 0.84 | |
| Median | 1.0 | |
| IQR | (0.7; 1.2) | |
| Range | (0.5; 4.5) | |
| MELD | N | 20 |
| Mean | 10.9 | |
| Std | 4.5 | |
| Median | 10.2 | |
| IQR | (7.8; 13.5) | |
| Range | (3; 20) |
N: number of observations. Std: standard deviation. IQR: interquartile range. ALT: alanine aminotransferase. INR: international normalised ratio. MELD: Model for End-Stage Liver Disease.
The mean PSPG before shunt creation was 15 mmHg (range 8-18 mmHg). The time interval between TIPS placement and reduction was 14 days (range 2-44 days).
The indication for shunt reduction was a twofold or more increase in bilirubin levels (n = 12; 57%) and/or a 50% increase in INR (n = 3; 14%) associated with clinical symptoms of grade 3-4 hepatic encephalopathy (n = 18, 88%).
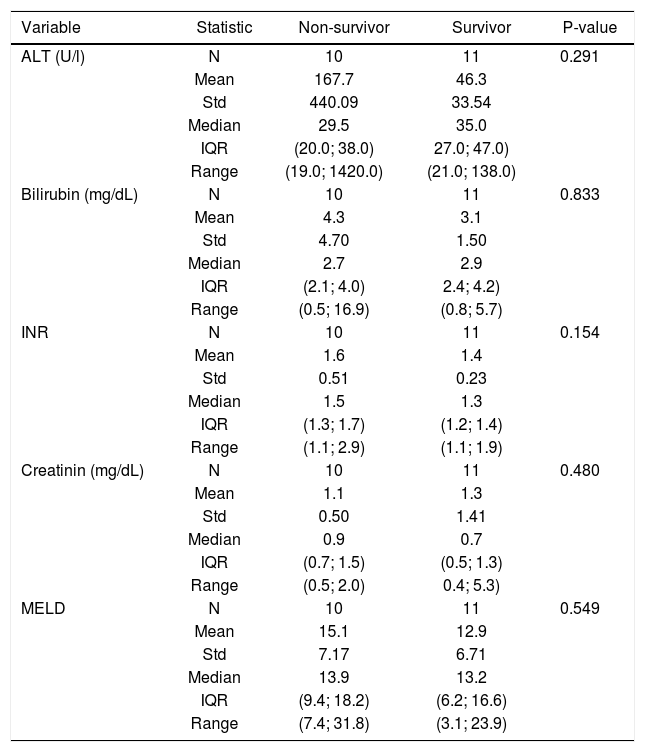
Depending on the general status of the patient, the shunt reduction procedure was performed under local (n = 17; 81%) or general (n = 4; 19%) anaesthesia. The laboratory values before and after the shunt reduction procedure are summarised in tables 2 and 3.
Laboratory values immediately before TIPS reduction.
| Variable | Statistic | Non-survivor | Survivor | P-value |
|---|---|---|---|---|
| ALT (U/l) | N | 10 | 11 | 0.291 |
| Mean | 167.7 | 46.3 | ||
| Std | 440.09 | 33.54 | ||
| Median | 29.5 | 35.0 | ||
| IQR | (20.0; 38.0) | 27.0; 47.0) | ||
| Range | (19.0; 1420.0) | (21.0; 138.0) | ||
| Bilirubin (mg/dL) | N | 10 | 11 | 0.833 |
| Mean | 4.3 | 3.1 | ||
| Std | 4.70 | 1.50 | ||
| Median | 2.7 | 2.9 | ||
| IQR | (2.1; 4.0) | 2.4; 4.2) | ||
| Range | (0.5; 16.9) | (0.8; 5.7) | ||
| INR | N | 10 | 11 | 0.154 |
| Mean | 1.6 | 1.4 | ||
| Std | 0.51 | 0.23 | ||
| Median | 1.5 | 1.3 | ||
| IQR | (1.3; 1.7) | (1.2; 1.4) | ||
| Range | (1.1; 2.9) | (1.1; 1.9) | ||
| Creatinin (mg/dL) | N | 10 | 11 | 0.480 |
| Mean | 1.1 | 1.3 | ||
| Std | 0.50 | 1.41 | ||
| Median | 0.9 | 0.7 | ||
| IQR | (0.7; 1.5) | (0.5; 1.3) | ||
| Range | (0.5; 2.0) | 0.4; 5.3) | ||
| MELD | N | 10 | 11 | 0.549 |
| Mean | 15.1 | 12.9 | ||
| Std | 7.17 | 6.71 | ||
| Median | 13.9 | 13.2 | ||
| IQR | (9.4; 18.2) | (6.2; 16.6) | ||
| Range | (7.4; 31.8) | (3.1; 23.9) |
ALT: alanine aminotransferase. INR: international normalized ratio. MELD: Model for End-Stage Liver Disease. N: number of observations. Std: standard deviation. IQR: interquartile range.
Peak laboratory data ten days after TIPS reduction.
| Variable | Statistic | Non-survivor | Survivor | P-value |
|---|---|---|---|---|
| ALT (U/l) | N | 10 | 11 | 0.597 |
| Mean | 133.7 | 42.7 | ||
| Std | 221.47 | 18.09 | ||
| Median | 43.0 | 45.0 | ||
| IQR | (28.0; 62.0) | 27.0; 48.0) | ||
| Range | (23.0; 613) | (23.0; 87.0) | ||
| Bilirubin (mg/dL) | N | 10 | 11 | 0.275 |
| Mean | 7.7 | 3.6 | ||
| Std | 7.85 | 1.85 | ||
| Median | 4.2 | 3.2 | ||
| IQR | (2.5; 10.2) | 2.3; 6.1) | ||
| Range | (2.1; 24.1) | (1.4; 6.4) | ||
| INR | N | 10 | 11 | 0.044 |
| Mean | 1.8 | 1.5 | ||
| Std | 0.44 | 0.23 | ||
| Median | 1.7 | 1.5 | ||
| IQR | (1.5; 2.2) | (1.3; 1.5) | ||
| Range | (1.3; 2.7) | 1.1; 2.0) | ||
| Creatinin (mg/dL) | N | 10 | 11 | 0.170 |
| Mean | 1.4 | 1.3 | ||
| Std | 0.60 | 1.31 | ||
| Median | 1.3 | 0.7 | ||
| IQR | (0.9; 1.8) | (0.6; 1.5) | ||
| Range | (0.5; 2.4) | 0.5; 5.1) | ||
| MELD | N | 10 | 11 | 0.062 |
| Mean | 19.8 | 14.1 | ||
| Std | 6.14 | 6.19 | ||
| Median | 19.0 | 13.7 | ||
| IQR | (14.2; 25.2) | (10.2; 19.2) | ||
| Range | (10.0; 29.6) | 3.3; 25.5) |
ALT: alanine aminotransferase. INR: international normalized ratio. MELD: Model for End-Stage Liver Disease. N: number of observations. Std: standard deviation. IQR: interquartile range.
The shunt reduction procedure was technically successful in all cases. Pressure measurements in the inferior vena cava and main portal vein, as well as the assessment of the PSPG and MELD scores for both survivors and non-survivors are summarised in table 4. The median time interval between TIPS placement and TIPS reduction was 10 (3-34) days in surviving patients and 20 (2-44) days in non-surviving patients (p-value = 0.1).
Pressure measurements before and after TIPS reduction.
| Variable | Statistic | Non-survivor | Survivor | P-value |
|---|---|---|---|---|
| PSPG before TIPS reduction (mmHg) | N | 10 | 11 | 0.523 |
| Std | 3.21 | 4.49 | ||
| Median | 7.5 | 9.0 | ||
| IQR | (5.0; 10.0) | 5.0; 13.0) | ||
| Range | (3.0; 13.0) | (3.0; 17.0) | ||
| Pressure in the inferior vena cava before TIPS reduction (mmHg) | N | 10 | 11 | 0.158 |
| Mean | 10.8 | 4.9 | ||
| Std | 8.56 | 3.48 | ||
| Median | 13.0 | 4.0 | ||
| IQR | (3.0; 17.0) | 2.0; 7.0) | ||
| Range | (−2.0; 23.0) | (1.0; 12.0) | ||
| Pressure in the main portal vein before TIPS reduction (mmHg) | N | 10 | 11 | 0.168 |
| Mean | 18.3 | 13.3 | ||
| Std | 10.44 | 4.86 | ||
| Median | 20.0 | 13.0 | ||
| IQR | (10.0; 27.0) | 9.0; 19.0) | ||
| Range | (3.0; 31.0) | 7.0; 20.0) | ||
| PSPS after TIPS reduction (mmHg) | N | 9 | 11 | 0.252 |
| Mean | 12.0 | 15.1 | ||
| Std | 4.90 | 5.56 | ||
| Median | 11.0 | 15.0 | ||
| IQR | (8.0; 15.0) | 11.0; 21.0) | ||
| Range | (7.0: 22.0) | 7.0; 23.0) | ||
| Pressure in the inferior vena cava after TIPS reduction (mmHg) | N | 9 | 11 | 0.039 |
| Mean | 11.1 | 5.0 | ||
| Std | 8.13 | 4.43 | ||
| Median | 12.0 | 6.0 | ||
| IQR | (7.0; 17.0) | (1.0; 7.0) | ||
| Range | (−5.0; 22.0) | (−1.0; 12.0) | ||
| Pressure in the main portal vein after TIPS reduction (mmHg) | N | 9 | 11 | 0.401 |
| Mean | 23.1 | 19.3 | ||
| Std | 11.37 | 5.37 | ||
| Median | 21.0 | 18.0 | ||
| IQR | (15.0; 31.0) | 15.0; 24.0) | ||
| Range | (3.0; 40.0) | 13.0; 29.0) | ||
| Time interval between TIPS and shunt reduction, days | N | 10 | 11 | 0.27 |
| days | 20 (2-44) | 10 (3-14) |
PSPG: portosystemic pressure gradient.
In all cases, angiographic evaluation before shunt reduction demonstrated a fully patent TIPS stent graft without opacification of any intrahepatic portal vein end branches; after shunt reduction there was antegrade reopacification of the intrahepatic portal vein branches in 13 patients (62%), i.e. in eight (73%) survivors and in five (50%) non-survivors.
In 5/21 (24%) patients additional coil embolisation of reopacified varices was performed.
During follow-up, reduced shunt occlusion occurred in two patients, resulting in recurrent refractory ascites in both patients. The occluded and reduced TIPS shunt was reopened using interventional techniques.
All patients were monitored until death (n = 13; 62%), liver transplantation (n = 4; 19%), or time of data analysis (n = 4; 19%). A total of 11 out of 21 patients (52%) were able to be discharged from the intensive care unit after a median of 10.6 days (range: 3-25 days). The overall one and six-month survival rates were 15/21 (71%) and 11/21 (52%), respectively. Four of the surviving patients (19%) underwent liver transplantation, respectively at 55, 56, 105 and 510 days after the shunt reduction procedure.
DiscussionThis study confirms the technical feasibility and reproducibility of the parallel technique to reduce the diameter of a portosystemic shunt in patients suffering from TIPS-induced acute liver decompensation, as previously demonstrated for the management of TIPS-induced hepatic encephalopathy.7–10 In line with previous reports,9,10 we also found quite a low reocclusion rate of 10% during follow-up; however, the follow-up period was relatively short, mainly due to the high early mortality rate in this patient population. In the case of reduced shunt occlusion, it is still technically feasible to recanalise and reopen the occluded shunt and reline with another ePTFE-covered stent graft.10
More importantly, the clinical outcome for patients suffering from TIPS-induced acute liver decompensation is very poor, with one and six-month survival rates ofjust under 70% and 50%, respectively, despite maximum medical and interventional management. TIPS-induced acute liver decompensation is a rare disease, with an incidence of 6% in this series, and might be considered as another form of acute-on-chronic liver failure (ACLF): all patients suffered from underlying liver cirrhosis with severe clinical signs of portal hypertension, and shortly after TIPS placement these patients developed acute liver decompensation with typical signs of ascites, encephalopathy, jaundice and other laboratory findings indicating liver failure.2–4 It is still unclear what the overall survival rate in patients with post-TIPS acute liver decompensation would be without interventional shunt-reduction treatment: no reports on the natural outcome of this disease exist in the literature. Moreau, et al.3 analysed the outcomes for a patient population suffering from ACLF not related to TIPS insertion and treated through medical management, and found a transplant-free mortality of 34% and 51% after 30 and 90 days, respectively, which is no different from our results after TIPS shunt reduction. Although a prospective multicentre study randomising patients between interventional shunt reduction and maximised conservative treatment should provide an answer to this question, such a study design seems to be very difficult in terms of recruiting patients, not only due to the rarity of the disease, but also because most of the patients will favour an interventional treatment as shunt reduction/ closure might return them to their pre-TIPS status. It is also clear that several pre-TIPS factors are prognostic for early post-TIPS mortality, such as a high MELD score,12–14 or Child-Pugh score.14 However, we did not identify any prognostic pre-reduction TIPS factors for improved survival once a patient had developed TIPS-induced acute liver decompensation. Conversely, increased INR values post-TIPS-reduction (p = 0.044) seem to be predictive of worse outcomes. There was also a tendency (p = 0.06) towards higher post-reduction MELD scores in the non-surviving group (MELD score 19.8) compared to the surviving group (MELD score 14.1). In a larger study population, this trend might potentially be significant. Another point of interest is the management of patients with post-TIPS severe liver function disturbances without clear clinical signs of liver failure, including ascites and hepatic encephalopathy. We encountered 3 patients with severe liver function disturbances post-TIPS and decided to reduce the TIPS in order to avoid later clinical complications of liver failure, although it is not clear if these clinical symptoms may occur later on. Last, we also found a post-TIPS-reduction increase in measured inferior vena cava pressure as another prognostic factor for early death. These data might be explained by the multi-organ failure status of most of these patients at the time of the shunt reduction procedure.
In conclusion, this study shows high one-month and six-month mortality rates of 30% and 50%, respectively in patients undergoing TIPS reduction for TIPS-induced acute liver decompensation. Increased levels of INR after TIPS reduction seem to be the most important prognostic parameter for early death.