Pulmonary hypertension is a common finding in patients with advanced liver disease. Similarly, among patients with advanced pulmonary arterial hypertension, right heart failure leads to congestive hepatopathy. Diuretic resistant fluid overload in both advanced pulmonary hypertension and chronic liver disease is a demanding challenge for physicians. Venous congestion and ascites-induced increased intra-abdominal pressure are essential regarding recurrent hospitalization, morbidity and mortality. Due to impaired right-ventricular function, many patients cannot tolerate extracorporeal ultrafiltration. Peritoneal dialysis, a well-established, hemodynamically tolerated treatment for outpatients may be a good alternative to control fluid status. We present a patient with pulmonary arterial hypertension and congestive hepatopathy hospitalized for over 3 months due to ascites induced refractory volume overload treated with peritoneal ultrafiltration. We report the treatment benefits on fluid balance, cardiorenal and pulmonary function, as well as its safety. In conclusion, we report a case in which peritoneal ultrafiltration was an efficient treatment option for refractory ascites in patients with congestive hepatopathy.
Removal of fluid overload is a challenge in the management of severe pulmonary hypertension (PH) and congestive hepatopathy in patients with ascites and diuretic resistance. We present the case of a recurrent hospitalized patient with refractory volume overload treated with peritoneal ultrafiltration.
Case ReportIn 2007, a 51-year-old Caucasian woman was admitted to our department diagnosed with severe idiopathic PH (mPAP 59 mmHg, PVR 900 dyn x sec x cm-5, PCWP 11 mmHg, CO 4,87 L/min). She was cyanotic, with oxygen saturation of 82%. Treated with sequential pulmonary vasoactive therapy (sildenafil, terguride, iloprost inhalation and ambrisentan), she was readmitted since 2011 every 2-3 months due to severe fluid overload (despite high doses of sequential nephrone blockade). The renal function decreased progressively with an estimated glomerularfiltration rate (eGFR using MDRD) of 41 mL/min and serum creatinine (SCr) of 140,8 μmol/l. Estimation of other renal biomarkers or collection of 24 h urine specimen was not performed. She has a weight gain of 30 kg from overhydration with severe edema and ascites. The ascites was considered due to congestive hepatopathy by chronic right heart failure (HF) with a Child-Pugh-Score of 8. No alcoholic intoxication was documented, the hepatitis B and C virus serology was negative and we excluded autoimmune disease and Budd-Chiari syndrome. We refrained from performing paracentesis due to abnormal prothrombin time, BMI of 51.9 m/kg2 and caput medusae. Initially, the symptoms of hypervolemia were relieved by intravenous (IV) furosemide administration. In later stages with decreasing renal function, treatment options were bailed out.
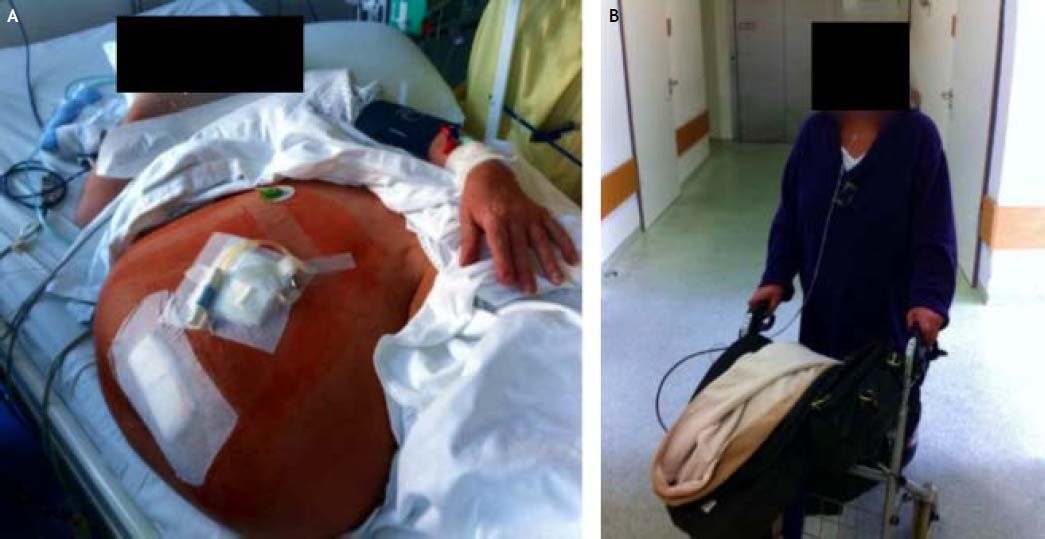
End of 2011, the oliguric patient was hospitalized on intensive care unit (despite IV furosemide and xipamide high doses), being dependent on IV iloprost and continuous extracorporal renal replacement therapy for 15 days. She lost 8 kg of fluid, resulting in a partial renal recovery. Some weeks later, staying on ICU for 3 months with the same therapeutic plan, this time without significant weight loss (Figure 1A). Her Karnofsky score remained 20% over the last 8 months.
The placement of a peritoneal dialysis (PD) catheter to mobilize ascites was discussed in the past but refused due to an excessive risk of general anesthesia. Regarded as a “lost case” the surgical department implanted a PD catheter in local anesthesia. During 15 days, 25 L of amber-colored ascites was drained (Figure 2). Laboratory testing revealed transudative ascites, rich in epithelial cells without signs of infection or malignancy. No extracorporeal treatment or diuretics (besides spironolactone because of hypokalemia) were necessary as diuresis increased. The eGFR was 38 mL/min with a SCr of 158 μmol/l and urine output about 2 L/day. She was no longer bed-ridden, oxygen supply could be reduced and the assessment showed a patient in no distress and without dyspnea at rest. The Child-Pugh-Score remained the same.
One hallmark of PD is the variable and individualized use of its solutions. At this point, when ascites decreased below 400 mL, peritoneal ultrafiltration was started with empirically 1,000 mL/day icodextrin and a dwell time of 24 h. The underlying rationale was to achieve as much UF as possible to avoid relapse, but use as less icodextrin as necessary in order to preserve residual renal function. For that, the daily amount of 400 mL ascites seemed reasonable to start with peritoneal ultrafiltration. The recommended dwell time for icodextrin is 8- to 16 h. However, a shorter period than 24 h and a larger fill volume did not significantly increase UF, so that we continued the abovementioned PD regime with that we could mobilize additional 15 L of body fluid. Hypoalbuminemia did not occur within the next months (lowest level 3.8 g/dL). Before dismissal, diuresis was 1.5 L (torasemide 20 mg, xipamide 20 mg, spironolactone 75 mg) and a peritoneal ultrafiltration 1 L/day. We saw her first on a weekly, then on a monthly basis, and Karnofsky index of 60% was reached (Figure 1B).
No hospital admissions were required for 1 year. Considering the lack of interpretability of eGFR and SCr under daily use of icodextrin, all attempts failed to measure urinary creatinine clearance. In November 2012, the PD treatment regime was adjusted to nocturnal continuous cycling peritoneal dialysis (CCPD) due to decreasing renal function with end stage renal disease (ESRD) and anuria with a total UF of 1,500 mL/day.
In 2013, she got hospitalized due to upper gastrointestinal bleeding based on congestive gastropathy. She was readmitted for the last time in March 2014 due to refractory terminal right HF. Clinical and hemodynamic assessments (fluid challenge and aggravation during UF, performing pulmonary arterial catheterization) revealed adequate fluid balance. The IV administration of sildenafil and epoprostenol did not lower the severity and laboratory studies showed no signs of infection or other causes. The patient died as a part of the diseases’ nature without any options left.
DiscussionRegarding the clinical scenario of both, PH and chronic liver disease, this case provides different teaching points. It underscores that refractory ascites can be successfully controlled with peritoneal ultrafiltration, even in non-ESRD. Functional renal impairment played an essential role, since renal function partially recovered. Right-sided HF and especially ascites-induced intra-abdominal hypertension can contribute to impaired kidney function.1–3 Patients with right-sided HF and hepatorenal syndrome 2 often present diuretic resistance due to venous congestion and hyperaldosteronism.4 Here, the paracentesis and administration of mineralcorticoid antagonists due to PD induced hypokalemia presumably provided escape from the vicious cycle, as she lost 30 kg of fluid. Since fluid overload can lead to acute respiratory failure and worsen pulmonary hypertension, we found respiratory function improvement after peritoneal ultrafiltration a keypoint in this case report.5–7 The success has to be defined in terms of reduced hospitalization numbers, improved mobility and quality of life. We avoided the implantation of transjugular intrahepatic portosystemic shunts, as the hemodynamic effects could have worsened PH.8,9
Second, similar to congestive HF, peritoneal ultrafiltration seems to be a favorable modality in PH, if refractory to diuretics.10 While extracorporal UF is fast and commonly available in acute setting, HF-patient suffers from immobility, potential hemodynamic fluctuations, risk of infection, bleeding, need for hospitalization, higher hospital expense and compromised quality of life.11–14 Patients with PH present strong hemodynamic fluctuations having a narrow window for fluid balance, as extremes can be associated with worsened renal and right ventricular function. Although no invasive hemodynamic measurements were performed after the initiation of peritoneal ultrafiltration, we can only predict the beneficial effect of fluid management in the progression of PH, since there was a significant clinical improvement and decrease of oxygen demand. Brain natriuretic peptide and sodium did not show any correlation with the clinical course.
We choose a therapeutic manoeuvre that has been reported to be useful and safe in patients with different diagnosis but similar clinical features. There are no randomized trials concerning the safety of PD catheter placement in patients with ascites due to PH, but clinical reports on its safety on cirrhotic patients.15 Technical survival of PD in cirrhosis has been described as long as 2 years.16 The case thought us to be careful in judging treatment as futile when quality of life is the ultimate goal.
Ours may be the first to report the application of peritoneal ultrafiltration in the treatment of refractory ascites in patient in chronic liver disease and PH. Given the lack and difficulty of randomization, this case report should help foster the notion of a multidisciplinary approach in PH.
SupportNone.
Financial DisclosureNone.