Lipoprotein X (LpX) is an abnormal lipoprotein associated with cholestasis. It is a significant cause of severe hypercholesterolemia and should always be considered in patients with cholestatic liver disease. This case highlights the significance of LpX as a cause of severe hypercholesterolemia in a patient with cholestasis secondary to a granulomatous hepatitis attributed to tuberculosis. Lipoprotein agarose gel electrophoresis and gradient gel electrophoresis were performed for the detection of LpX. The liver function tests, electrolytes, lipid profile and bile acids were also determined. Anti-tuberculous therapy was initiated and the liver functions improved with normalisation of the lipid profile.
Lipoprotein X (LpX) is an abnormal lipoprotein rich in phospholipids and unesterified cholesterol with albumin as the main protein. It was first decribed in the 19th century in patients with obstructive jaundice and elevated serum lipids. Further studies attributed this to the presence of an abnormal low density lipoprotein (LDL); however this abnormal lipoprotein was only isolated and characterized in the late sixties in patients with biliary obstruction.1,2 It was also found that experimentally induced cholestasis in animals led to a rapid increase in LpX which normalised within a week after alleviation of cholestasis.3 It was suggested that LpX originated from reflux of bile lipoprotein into the plasma leading to decreased catabolism of this intestinal lipoprotein.2,3 We present a case of severe hypercholesterolemia in a patient with cholestatic liver disease.
Case ReportA 46 year old female patient was referred from her local clinic to Tygerberg Hospital in February 2014 with a history of persistently deranged liver function tests since October 2013. She was known with human immunodeficiency virus 1 (HIV-1) since 1997 and has been on co-trimoxazole (Bactrim) therapy since 1998. She is a known hypertensive on enalapril and has a previous history of abdominal tuberculosis (TB), having completed her anti-TB therapy in May 2013. Anti-retroviral therapy (ART) was initiated in August 2013 consisting of efavirenz (EFZ), lamivudine (3TC) and zidovudine (AZT). At the time of initiation of ART her latest CD4 T cell count was 510 (cells/mm3).
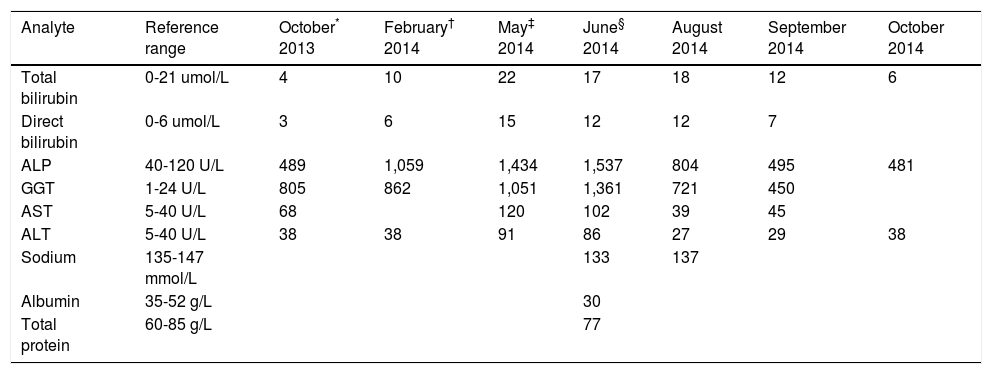
She was followed up monthly since August 2013 at the clinic and her full blood count, liver and renal functions were monitored. In October 2013, for unknown reasons, a full liver function test was performed and a cholestatic picture with raised alkaline phosphatase (ALP) and gamma glutamyl-transferase (GGT) but no hyperbilirubinaemia was detected. The cholestasis deteriorated over the next 3 months (Table 1). Hepatitis studies were performed in January 2014 and were found to be negative for Hepatitis B and C. The patient was then referred to the infectious diseases clinic at Tyger-berg Hospital.
Serum biochemistry results.
| Analyte | Reference range | October* 2013 | February† 2014 | May‡ 2014 | June§ 2014 | August 2014 | September 2014 | October 2014 |
|---|---|---|---|---|---|---|---|---|
| Total bilirubin | 0-21 umol/L | 4 | 10 | 22 | 17 | 18 | 12 | 6 |
| Direct bilirubin | 0-6 umol/L | 3 | 6 | 15 | 12 | 12 | 7 | |
| ALP | 40-120 U/L | 489 | 1,059 | 1,434 | 1,537 | 804 | 495 | 481 |
| GGT | 1-24 U/L | 805 | 862 | 1,051 | 1,361 | 721 | 450 | |
| AST | 5-40 U/L | 68 | 120 | 102 | 39 | 45 | ||
| ALT | 5-40 U/L | 38 | 38 | 91 | 86 | 27 | 29 | 38 |
| Sodium | 135-147 mmol/L | 133 | 137 | |||||
| Albumin | 35-52 g/L | 30 | ||||||
| Total protein | 60-85 g/L | 77 |
ALP: alkaline phosphatase. GGT: γ-glutamyl transferase. AST: aspartate transaminase. ALT: alanine transaminase.
At the time of the initial presentation at Tygerberg Hospital in February 2014, the patient did not report any history of abdominal pain, constitutional symptoms or jaundice. On the physical examination, there was no jaundice, oral candidiasis and anemia. Her blood pressure was 180/100 mmHg, pulse rate 82, capillary glucose 4.7 mmol/l and her body mass index 21.8 kg/m2. There was no evidence of hepatomegaly on abdominal examination and the rest of the systemic examination was within normal limits. A provisional diagnosis of steatohepatitis secondary to Zidovudine was made. An abdominal sonar was requested and bloods were collected for liver function tests (Table 1). The co-trimoxazole was stopped and her ART was changed to the standard first line therapy consisting of efavirenz (EFZ), emtracitabine (FTC), tenofivir (TDF).
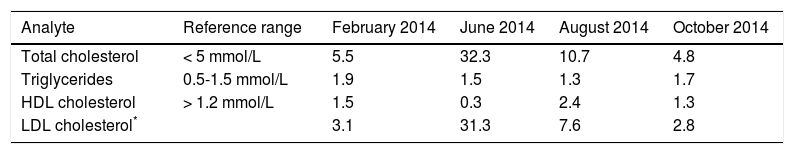
One week later, at the follow up visit, the patient did not report any problems on the new regimen. A lipogram was requested and the total cholesterol was 5.5 mmol/l with a triglyceride value of 1.9 mmol/l (Table 2). It is not clear if the patient was fasting at the time of blood collection. The abdominal sonar demonstrated a fatty infiltration of the liver.
Lipid profile results.
| Analyte | Reference range | February 2014 | June 2014 | August 2014 | October 2014 |
|---|---|---|---|---|---|
| Total cholesterol | < 5 mmol/L | 5.5 | 32.3 | 10.7 | 4.8 |
| Triglycerides | 0.5-1.5 mmol/L | 1.9 | 1.5 | 1.3 | 1.7 |
| HDL cholesterol | > 1.2 mmol/L | 1.5 | 0.3 | 2.4 | 1.3 |
| LDL cholesterol* | 3.1 | 31.3 | 7.6 | 2.8 |
HDL: high density lipoprotein. LDL: low density lipoprotein.
The patient was followed up monthly and over the next three months the liver enzymes progressively worsened with development of hyperbilirubinemia and mildly elevated transaminases (Table 1). The liver biopsy, in May 2014, revealed a granulomatous hepatitis with focal necrotising granulomas, associated fibrosis and portal tract fibrosis. Hepatitis B and C serology was repeated and it remained negative. Her blood pressure was poorly controlled and a second antihypertensive agent i.e. amlodipine was added.
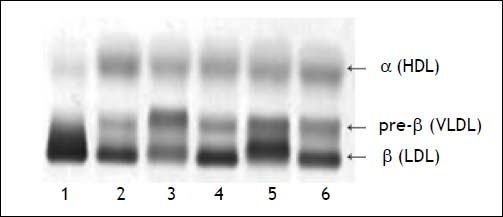
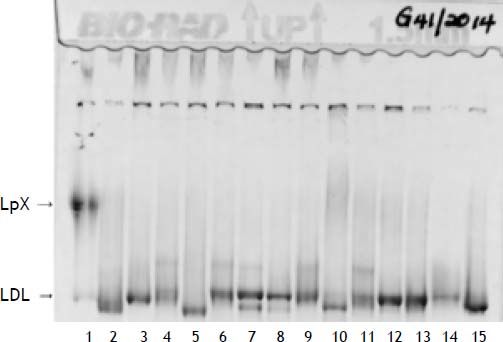
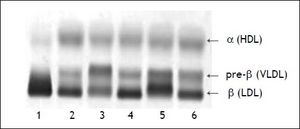
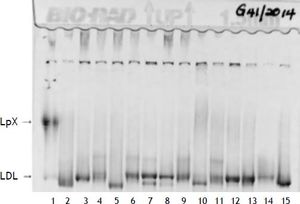
Two weeks following the biopsy, a severe hypercholesterolemia was detected with a total cholesterol value of 32.3 mmol/l (Table 2). On physical examination there was no evidence of xanthelasma, arcus or tendon xanthomata that may suggest familial hypercholesterolemia. Hypothyroidism and nephrotic syndrome were excluded, based on the absence of clinical signs with normal thyroid stimulating hormone and urine protein respectively. The severe hypercholesterolemia was thought to be due to LpX and lipoprotein agarose gel electrophoresis was performed. The lipoprotein agarose gel electrophoresis was not diagnostic of LpX. There was intense staining of the beta area with minor cathodic migration and the beta band had a slight green tinge (Figure 1). However, LpX was demonstrated by gradient gel electrophoresis (Figure 2). The bile acids were determined and found to be increased. The 3α-hydroxy sterol concentration in our patient was 322.3 μmol/l (reference range < 10 μmol/l).
Polyacrylamide non-denaturing gradient gel lipoprotein electrophoresis. Lane 1, patient sample. The LpX band is of narrower size range than VLDL and often found in the mid region (as above). The LDL species in this patient of intermediate size and very low concentration (estimate of < 0.5 mmol/L).
A repeat liver biopsy was performed in June 2014 with the intention to send a specimen to Microbiology for TB culture. However, the sample was only sent to histopathology. The liver biopsy showed a granulomatous hepatitis and the stains were negative for TB, fungal spores and hyphae, amyloidosis, iron and copper deposition. Although there was no clinical evidence of sarcoidosis, determination of angiotensin converting enzyme (ACE) was done to exclude sarcoidosis. The ACE concentration was below the detectable limit (< 8 U/l). Blood, sputum and urine cultures were all negative for TB. However, the patient was started on empiric TB treatment in June 2014, and this resulted in a significant improvement of liver function tests (Table 1) and normalisation of the lipid profile (Table 2). She also had a mild hyponatremia which has now normalised. She continues to follow up monthly.
DiscussionHypercholesterolemia is a common finding in adults and it is one of the major causes of coronary artery disease. Most cases of hypercholesterolemia are multifactorial with obesity, a high fat diet and an underlying polygenic predisposition as the major contributors. Hypercholesterolemia is typically categorised into primary and secondary causes. Primary causes of hypercholesterolemia are due a genetic component and include conditions like familial hypercholesterolemia (FH).4 Secondary causes of hypercholesterolemia must always be excluded. The major causes are hypothyroidism, nephrotic syndrome and liver disease. In such cases therapy is directed towards the primary disorder however statin therapy or plasma apheresis may be required in some patients.4,5
The liver plays an important role in lipid metabolism. Therefore, abnormal liver functions can result in abnormal plasma lipids, lipoproteins and apolipoproteins.6 Hypercholesterolemia associated with liver disease was first described by Flint in 1862, in patients with obstructive jaundice. However, the mechanisms or the lipoprotein responsible for the elevated cholesterol levels was not known. In 1969, Siedel, et al. designated this lipoprotein associated with biliary obstruction as LpX. The lipoprotein pattern of these patients was characterised by an increased LDL concentration and a decreased high density lipoprotein (HDL) concentration.1
LpX is a low density lipoprotein and it is made up largely of phospholipids (66 %) and unesterified cholesterol (22%) with very little triglyceride (3%), cholesterol ester (3%) and protein content (6%). The protein component of LpX is made up of albumin found within its core, which makes up to 60% of the protein content, and apolipoprotein C located on the surface.1,3,6 Despite the characterization of LpX, much was not known about its metabolism. It was postulated that LpX originated from intestinal mucosa and is normally undetectable in the blood during the fasting state, due to the rapid degradation in the liver. In obstructive jaundice, the increase in LpX was thought to be due to increased bile acids resulting in inhibition of catabolic reactions of LpX.1
LpX is found in various liver diseases associated with cholestasis. It has been found, amongst others, in primary biliary cirrhosis, bile duct obstruction, chronic grafft-vs.-host disease of the liver as well as viral and drug induced hepatitis.1,2,7,8
A literature search revealed that hypercholesterolemia in granulomatous hepatitis has only been described once in two cases written up in 1976. However, in both cases the hypercholesterolemia was not as severe as in our patient; it developed a few years following a splenorenal shunt procedure and resolved on a low cholesterol diet. The lipoprotein electrophoresis of both patients showed a Type IIa pattern i.e. an abnormal increase in LDL.9
Unlike LDL, LpX is not taken up by the liver; it is instead cleared by the reticuloendothelial system particularly the spleen. Therefore, it is unable to exert negative feedback on the cholesterol synthesis rate limiting enzyme hydroxymethyglutaryl coenzyme A (HMG-CoA) reductase. In contrast, the presence of LpX increases the activity of HMG-CoA reductase in the liver with increased hepatic cholesterol synthesis.6,10
Pseudohyponatremia has been described in cases of increased LpX.11,12 This falsely decreased sodium concentration occurs when sodium is measured on an analyser that uses indirect ion-selective electrode potentiometry. A normal measured serum osmolality in the face of hyponatremia should point to the presence of pseudohyponatraemia. Measurement of sodium with an analyser that uses direct ion-selective electrode potentiometry, e.g. blood gas analyser, would be advised in patients with LpX.11 LpX has also been found to interfere with measured (direct) and calculated LDL cholesterol.13 Our patient had borderline decreased sodium which may have been due to this, but was never investigated. As the sodium levels returned to normal we presume there was an element of pseudohyponatremia.
Complications of LpX include development of xanthomata, retinal cholesterol, thromboembolism, cholesteroloma of the lung and hyperviscosity syndrome.7 Although cholesterol levels are extremely high in cases of raised LpX, patients with chronic cholestasis such as primary biliary cirrhosis do not have a significantly increased incidence of coronary artery disease. It has been described that LpX may in fact have an anti-atherogenic effect by decreasing LDL oxidation. This is thought to be due to the high phospholipid content of LpX. This protective effect decreased after liver transplantation and normalization of LpX levels.14 Patients with LpX do not usually require therapy, however very high concentrations of LpX can result in the above mentioned complications which require therapy. The first choice therapy in complicated cases of LpX is LDL apheresis.7,8,15
The presence of LpX can be detected on agar-gel electrophoresis. LpX migrates towards the cathode on agar-gel electrophoresis.1,3 Nuclear magnetic resonance spectroscopy, ultracentrifugation, chemical and immunological assays are alternative methods for the determination of LpX.1,3,13,15 We failed to demonstrate the presence of LpX on agarose gel elec-trophoresis; however it was confirmed on gradient gel electrophoresis.
ConclusionWe describe an unusual case of severely increased cholesterol levels in a patient with cholestasis due to the presence of LpX. The cholesterol levels normalised with improvement of the cholestasis. This patient also had decreased sodium levels which may be due to pseudohyponatremia associated with this condition. Although LpX was described over a century ago, there is a lack of understanding about its effects and clinicians often seem to miss this condition. Laboratory staff and clinicians need to be aware of the possible causes of increased LpX.
Abbreviations- •
ART: antiretroviral therapy.
- •
LDL: low density lipoprotein.
- •
LpX: lipoprotein X.
- •
TB: tuberculosis.
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Conflict of InterestThe authors declare that there are no conflicts of interest associated with this work.
AcknowlegementsWe would like to thank Dr. Hugo for the clinical information of the patient; UCT lipid laboratory staff for the determination of the gradient gel electrophoresis and bile acids; Prof. David Marais and Dr. Dirk Blom for the clinical input and interpretation of both the lipoprotein agarose and gradient gel electrophoresis.