Background. The plasminogen activator inhibitor type-1 (PAI-1) has been implicated in the regulation of fibrinolysis and extracellular matrix components. The single base pair guanine insertion/deletion polymorphism (4G/5G) within the promoter region of the PAI-1 gene influences PAI-1 synthesis and may modulate hepatic fibrogenesis.
Aim. To evaluate the influence of PAI-1 serum levels and 4G/5G polymorphism on the risk of liver fibrosis associated to non-alcoholic fatty liver disease (NAFLD) in morbidly obese patients.
Material and methods. Case-control study of 50 obese patients undergoing bariatric surgery and 71 non-obese subjects matched by age and sex. Anthropometric and biochemical measurements were performed, including PAI-1 serum levels. Genomic DNA was obtained to assess the presence of 4G/5G polymorphism.
Results. BMI, insulinemia, triglycerides, HOMA-IR, hypertension and diabetes were significantly higher in obese patients compared to control subjects. PAI-1 serum levels observed in obese patients were significantly lower (10.63 ± 4.82) compared to controls (14.26 ± 11.4; p < 0.05). No differences were observed in the PAI-1 4G/5G promoter genotypes frequencies (p = 0.12). No differences were observed in PAI-1 plasma levels among obese patients with liver fibrosis (10.64 ± 4.35) compared to patients without liver fibrosis (10.61 ± 5.2; p = 0.985). PAI-1 4G/5G promoter genotypes frequencies were similar in patients with or without liver fibrosis associated to NASH (p = 0.6).
Conclusions. Morbidly obese patients had significantly lower PAI-1 serum levels with similar PAI-1 4G/5G genotypes frequencies compared to non-obese subjects. The frequency of 4G/5G genotypes in Chilean Hispanic healthy subjects was similar to that described in other populations. No association was found between PAI-1 serum levels or 4G/5G genotype with liver fi-brosis in obese patients.
Obesity complications, as cardiovascular diseases, metabolic syndrome, diabetes, hypertension, dyslipi-demia, psychosocial disorders and gastrointestinal diseases are an important public health issue.1 Among the gastrointestinal complications non-alcoholic fatty liver disease (NAFLD) is commonly observed in clinical practice settings. Advanced features of this disease, such as non-alcoholic stea-tohepatitis (NASH), could evolve to cirrhosis and hepatocellular carcinoma.2 Other important aspect of obesity-related complications is the association of NAFLD with cardiovascular disease. The association of abdominal obesity and insulin resistance is considered a risk factor for cardiovascular disease.3–5 Furthermore, an association between obesity and reduced fibrinolytic activity contributes to a higher risk of cardiovascular events. This hypofibrinolysis is in part due to increased levels of human plasmi-nogen activator inhibitor type 1 (PAI-1).6–8
PAI-1 is a single chain glycoprotein of 48-kDA, member of the serpin protein family, which contains 379 amino acids. It is the main inhibitor of fibrinolysis, representing approximately 60% of its inhibition. The endothelium and smooth muscle cells represent the major sources of PAI-1, but it is also produced by other cells like platelets, hepatocytes, hepatic stellate cells, mesangial cells, fibroblasts, monocytes, macrophages, adipocytes and stromal cells of adipose tissue.9
Given the anti-fibrinolytic activity of PAI-1, it could also participate in the mechanisms of organ fi-brogenesis. PAI-1 tends to maintain the integrity of the extracellular matrix (ECM) preventing degradation of its components (fibronectin, laminin, proteo-glycans and type IV collagen), resulting in fibrosis when high levels of PAI-1 are observed.10 In PAI-1 gene knockout mice under bile duct ligation and CCl4, severity of fibrosis was minimal.11–12 The expression of PAI-1 gene, which is located in the 7q21.3-q22 chromosome, is primarily regulated at transcriptional level through the action of many hormones and cytokines such as:13–15
- •
Renin-angiotensin-aldosterone system.
- •
Pro-insulin.
- •
Insulin.
- •
Tumor necrosis factor alpha.
- •
Lipoproteins.
- •
Glucose.
- •
Endotoxins.
- •
Low levels of nitric oxide (NO).
- •
Low levels of adiponectin.
Polymorphisms that influence the expression of PA1-1 have been described,16 eight single nucleotide polymorphisms have been described in the PAI-1 gene. The insertion/deletion polymorphism (4G/5G) in the promoter region at position 675 has been linked to differences in PAI-1 transcriptional activation. The 4G allele is associated with enhanced gene expression as it binds to an activator protein, whereas the 5G allele contains an additional binding site for a DNA binding protein acting as a trans-criptional repressor.17
The presence of these polymorphisms and their clinical consequences varies according the ethnic background, and has been linked with hepatic fibro-sis,18 but their role has not been clarified completely in obesity and NAFLD.
ObjectiveTo evaluate the potential involvement of the PAI-1 serum levels and 4G/5G polymorphism in the development of liver fibrosis associated to NAFLD in Chilean (Hispanics) obese patients. Our hypothesis is that patients with the 4G/4G genotype of PAI-1 express higher serum levels of PAI-1 and higher frequency of liver fibrosis.
Material and MethodsPatients and study designA case-control study including morbidly obese patients undergoing bariatric surgery in our institution was conducted from 2004 to 2007. The study was approved by the Institutional Review Board Ethics Committee for Human Studies of the Ponti-ficia Universidad Católica de Chile and informed consent was obtained from all participants. Control subjects; body mass index (BMI) < 25, serum alanine aminotransferase (ALT) < 30 IU/L in men and ALT < 19 IU/L in women19 and an abdominal ultrasound without fatty liver, were recruited from an ancillary epidemiological study that studied the prevalence and natural history of cholelitiasis and NAFLD, in the South-Eastern urban area of Santiago, Chile,20–21 and were matched by sex and age. Abdominal ultrasound in control subjects were per-formed by 2 well-trained physicians using a 3.5 MHz linear transducer (Toosbee, Toshiba, Japan). Fatty liver was defined as liver parenchyma with echogenicity higher than the right kidney cortex on 2 different probe positions, presence of vascular blurring and deep attenuation.22–23 The screening protocol included a pre-coded questionnaire with socioeconomic data, medical history including pre-vious known diagnosis of hypertension, diabetes, liver diseases, a detailed history of current alcohol consumption with an estimation of daily intake in grams per day, and concomitant medication use. Morbidly obese patients and matched controls with known alcohol consumption over 20 g per day, and those with chronic hepatic disease of a known origin (chronic viral hepatitis, autoimmune hepatitis, drug-induced liver disease, primary biliary cirrhosis, hemochromatosis, Wilson’s disease, α-1 anti-trypsin-deficiency-associated liver disease) were excluded. Hispanic ethnicity was assessed using the Amerindian admixture index (AAI) based on the distribution of ABO blood group assuming a bipa-rental origin of a hybrid population. Additionally, the control group was studied using mitocondrial DNA to established the Hispanic origin and subjects with Amerindian origin were excluded.24–25 The population results were assessed according to the Hardy-Weinberg equilibrium.26
Clinical and biochemical measurementsBlood samples from each subject were obtained. Serum fasting glucose, ALT and lipid profile measurements were performed in an automatized Roche Hitachi Modular chemistry analyzer (Hitachi, Tokyo). Hepatitis C virus (HCV) antibodies were measured by a third-generation immunoassay test, using the MEIA (Microparticle Enzyme Immunoas-say) technique on the Abbott AxSYM™ (Abbott Park, IL). Serum PAI-1 levels were determined by ELISA technique (Quantikine™, R&D Systems, Minneapolis, MN). Insulin serum level was measured with the Immulite 2000 equipment with DPC reactive (Diagnostics Product Corporation, Los Angeles, CA). Insulin resistance was determined by the homeostasis model assessment-insulin resistance (HOMA-IR) method which has a strong correlation with the clamp method to determine total glucose disposal and to assess insulin sensitivity.27 HOMA-IR was calculated according to the formula: insulin (μU/mL) x fasting plasma glucose (mmol/L)/22.5. In Chile, a HOMA-IR value > 2.6 is indicative of insulin resistance in non-diabetic subjects according to Acosta, et al.28 Metabolic syndrome, defined according to ATP III criteria,29 was established when three or more of the following abnormalities were present:26
- •
Waist circumference > 102 cm in men and 88 cm in women.
- •
Serum triglycerides level ≥ 150 mg/dL (1.69 mmol/L).
- •
HDL cholesterol concentration < 40 mg/dL (1.04 mmol/L) in men and < 50 mg/dL (1.29 mmol/L) in women.
- •
Blood pressure ≥ 130/85 mmHg.
- •
Serum glucose level ≥ 110 mg/dL (6.1 mmol/L).
Abnormal aminotransferase levels were defined as ALT > 30 IU/L in men and ALT > 19 IU/L in women.30 Diabetes mellitus was defined using the American Diabetes Association criteria.31 Previously known hypertension was defined as blood pressure > 140/90 mmHg in two different measurements, known previous diagnosis or antihyperten-sive drugs use.32
All patients met the inclusion criteria for obesity surgery corresponding to a body mass index (BMI) > 40 kg/m2 or a BMI > 35 kg/m2 with significant comor-bid conditions such as arterial hypertension, type 2 diabetes mellitus, sleep apnea or dyslipidemia.33
Histological evaluation of liver biopsiesAn intraoperative liver biopsy specimen was obtained at the beginning of the surgical procedure in morbidly obese patients undergoing bariatric surgery. Liver biopsies were examined by a single pathologist (G.C.) using the classification for NAFLD developed by Kleiner, et al.34
Genotyping of polymorphismBlood sample for each individual were obtained, red blood cells were lysed with ammonium chloride 0.8% and genomic DNA was extracted using Wizard Genomic DNA Purification kit (Promega, Madison, WI). PAI-1 polymorphisms were analysed by PCR, in a termocycler PTC-100, MJ Research Inc. (Water-town, MA). Forward and reverse primers were as follows:35–36
- •
5'-CACAGAGAGAGTCTGGCCACGT-3' and
- •
5'-CCAACAGAGGACTCTTGGTCT-3', respectively.
Reaction conditions were: 1 min at 95 °C followed by 35 cycles of 45 sec at 95 °C, 45 sec at 61 °C, 45 sec at 72 °C and a final extension of 1 min at 72 °C. Then PCR product (100 bp) was digested with BselI (Fermentas Life Science) (Burlington, ON) 2 h at 55 °C. DNA fragments were separated by 4% agarose gel electrophoresis and classified in 3 genotypes: 4G for 98bp fragment, 5G for 77 and 23bp fragments and 4G/5G for 98, 77 and 23bp fragments.
Statistical analysisThe clinical characteristics and laboratory measurements of morbidly obese patients with subjects of the control group were compared using the Student's t-test for independent samples. Discrete variables were compared using chi-square and Fisher test. Contrast t-test was used to compared means and contrast Mann-Whitney to compare medians. All the continuous variables are presented as mean ± standard deviation (SD). Discrete variables are presented with percentages. HOMA-IR showed a non-parametric distribution and it was expressed in the results as median (interquartile range) and statistical analysis was performed using the Kruskal Wallis non-parametric test and chi-square for categorical data. All statistical analyses were performed with SPSS version 10.0 (standard version, SPSS Inc.). Morbidly obese were analyzed according to histological findings (i.e. normal histology, simple steatosis and NASH). Patients were also analyzed according to the presence or absence of liver fibrosis.
Univariate and multivariate analysis were performed comparing morbidly obese patients with or without liver fibrosis. Potential clinical, metabolic and biochemical variables associated with liver fi-brosis were examined by comparing means and proportions. To further study these relationships, uni-variate logistic regression analysis was performed. In order to identify independent variables associated with liver fibrosis, a stepwise procedure for a multivariate logistic regression analysis was carried out, which included variables that appeared significant in univariate analysis. Highly correlated variables were excluded to avoid multicollinea-rity (i.e. insulin and HOMA-IR). Statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC), R and S-PLUS (Insightful Corp. Seattle, WA) software. Odds ratio (OR) and 95% confidence intervals (CI) were calculated. Differences were considered significant with p-values < 0.05.
ResultsDescription of patients and controlsThe baseline characteristics of patients and controls were similar in age and sex. The morbidly obese group had a significantly higher BMI, insulin, HOMA-IR, triglycerides and a higher frequency of hypertension and diabetes mellitus. On the other hand, lower levels of HDL cholesterol were observed in patients compared to control group (Table 1). The type of bariatric surgery they underwent was mainly Laparoscopic Roux-en-Y gastric bypass (90%).
Baseline characteristics of obese patients and control group.
| Variable | Patients (n= 50) | Controls (n= 71) | p value |
|---|---|---|---|
| Age (y)* | 42.28 ± 10.23 | 42.27 ± 10.24 | 0.4857 |
| Sex (men/women)† | 13(26) / 37(74) | 15(21.1) / 56(78.9) | 0.8869 |
| BMI (kg/m2)‡ | 41.69 ± 6.3* | 22.92 ± 1.6 | 0.0001 |
| Serum glucose (mg/dL)‡ | 100.94 ± 28.88 | 87.00 ± 36.56 | 0.2631 |
| Insulin (uU/mL)‡ | 19.78 ± 14.73* | 8.8 ± 14.86 | 0.0001 |
| HOMA-IR§ | 4.1 (2.6 - 5.9)* | 1.85 (1.1 - 2.3) | 0.0001 |
| Total cholesterol (mg/dL)‡ | 181.9 ± 37.89 | 201.00 ± 39.78 | 0.7889 |
| LDL cholesterol (mg/dL)‡ | 127.20 ±41.10 | 128.15 ± 35.34 | 0.6695 |
| HDL cholesterol (mg/dL)‡ | 48.9 ± 11.06* | 53.52 ± 11.92 | 0.0032 |
| Triglycerides (mg/dL)‡ | 145 ± 70.68* | 93.00 ± 59.86 | 0.0001 |
| Hypertension†,|| | 17(34)* | 7(10) | 0.0010 |
| Diabetes mellitus† | 7(14)* | 2(3) | 0.0368 |
We determined the AAI of the morbidly obese patients and controls in study based on the distribution of ABO blood groups. In both groups the AAI was similar: 0.37 and 0.38, for obese patients and controls respectively (Table 2).
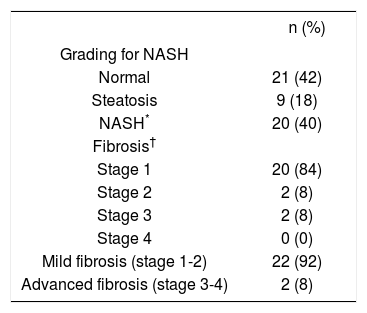
The histological analysis of liver biopsies performed in 50 morbidly obese patients according to the modified Brunt classification by Kleiner revealed that 42% (n = 21) had a normal liver, 18% (n = 9) simple steatosis, 40% (n = 20) NASH, in the last group of patients the majority was associated with fibrosis (n = 19). Of the total of 50 morbidly obese patients, 24 of them showed some degree of hepatic fibrosis, where 84% had perisinusoidal or portal fi-brosis (stage 1). By grouping the stages in mild fibrosis (stages 1 and 2) and advanced fibrosis (stage 3 and 4) it displayed 92 and 8%, respectively. The majority of patients (19 out of 24) have liver fibrosis associated to NASH (79%). However, 5 out of 24 (11%) showed mild and unspecific liver fibrosis (stage 1-2) (Table 3).
Grading and fibrosis staging for NASH according to Kleiner Classification in morbidly obese patients.
The study of the 4G/5G polymorphism of PAI-1 showed similar genotype and allele distribution in both groups (Table 4).
PAI-1 4G/5G polymorphism and allelic frequency in patients and control.
| 4G/5G polymorphism | Patients (n = 50) n (frequency, %) | Controls (n = 71) n (frequency, %) | p-value |
|---|---|---|---|
| Allele | |||
| 5G | 44 (88) | 52 (73) | 0.10 |
| 4G | 32 (64) | 51 (72) | - |
| Genotype | |||
| 5G/5G | 18 (36) | 20 (28) | - |
| 4G/5G | 26 (52) | 32 (45) | 0.12 |
| 4G/4G | 6 (12) | 19 (27) | - |
| Genotype | |||
| 5G/5G | 18 (36) | 20 (28) | 0.47 |
| 4G/4G + 4G/5G (presence of 4G allele) | 32 (64) | 51 (72) | - |
By comparing the genotype of patients with liver fibrosis associated to NASH and patients without fi-brosis, no significant differences were observed in the genotype of the 4G/5G polymorphism of PAI-1. Both groups showed similar distribution of alleles in the group with fibrosis and in the group without fi-brosis (Table 5).
PAI-1 4G/5G polymorphism and allelic frequency in patients with liver fibrosis associated to NASH.
| 4G/5G polymorphism | Fibrosis (n = 19) n (frequency, %) | Without fibrosis* (n = 31) n (frequency, %) | p-value |
|---|---|---|---|
| Allele | |||
| 5G | 17 (90) | 27 (87) | 0.91 |
| 4G | 12 (63) | 20 (65) | - |
| Genotype | |||
| 5G/5G | 7 (37) | 11 (35) | - |
| 4G/5G | 10 (52) | 16 (52) | 0.6 |
| 4G/4G | 2 (11) | 4 (13) | - |
| Genotype | |||
| 5G/5G | 7 (37) | 11 (35) | 0.93 |
| 4G/4G + 4G/5G (presence of 4G allele) | 12 (63) | 20 (65) | - |
Finally, by comparing the genotype of patients with NASH associated to liver fibrosis with those without liver fibrosis, no significant differences were observed in the genotype of the 4G/5G polymorphism of PAI-1 (data not shown). Both groups showed similar distribution of alleles in the group with fibrosis and in the group without fibrosis. The population was in Hardy-Weinberg equilibrium.
Determination of plasma levels of PAI-1PAI-1 serum levels observed in obese patients were significantly lower (10.63 ± 4.82) compared to controls (14.26 ± 11.4) with a p value < 0.05. The PAI-1 serum levels were not different among groups according the presence or absence of NAFLD. Even when we observe a trend to lower levels in subjects with NAFLD, particularly in those patients with genotype 5G/5G (Table 6), no differences were observed in PAI-1 serum levels among obese patients with liver fibrosis (10.64 ± 4.35) compared to those patients without liver fibrosis (10.61 ± 5.2) with a p value = 0.985.
Serum levels of PAI-1 according PAI-1 gene polymorphism and fatty liver status.
| All patients | 4G/4G | 5G/5G | 4G/5G | |
|---|---|---|---|---|
| Controls | 13.1 (2-95.3) | 12.9 (2-31.9) | 13.1 (2-95.3) | 13.8 (6-25.8) |
| NAFLD | 10.9 (1.8-23.9) | 11.2 (6.1-19.9) | 9.6 (1.8-18.7) | 11.5 (7.5-23.9) |
Median (minimum-maximum) serum level of PAI-1 in ng/mL. No significant differences were found comparing serum levels of PAI-1 according to PAI-1 gene polymorphism in patients with NAFLD and controls.
The PAI-1 promoter 4G/5G insertion/deletion polymorphism has been associated with a variable plasma activity of PAI-1 and its serum levels. The frequencies of genotypes 4G/4G, 4G/5G, and 5G/5G are in average approximately 25, 50 and 25% respectively, in various series.37 The 4G/4G genotype has a lower affinity for the transcription factor suppressor and individuals carrying these variants have an increased PAI-1 gene expression, as well as increased PAI-1 plasma levels. Subjects homozygous for the 4G allele (4G/4G genotype) have plasma levels of PAI-1 approximately 25% higher than subjects who are homozygous for the 5G allele (5G/5G genoty-pe).37–38 Since PAI-1 levels has been related to fibro-sis in other organs,39–40 we genotyped a group of morbidly obese patients with high risk for obesity-associated liver fibrosis linked to underlying NASH.
No significant differences were found in the genotype of the 4G/5G polymorphism of the PAI-1 gene among a group of morbidly obese patients and control subjects, proving a similar AAI, given the bipa-renteral origin of our population (Hispanic and Mapuche). We did not find a higher frequency of 4G/ 4G genotype in morbidly obese patients, which is in agreement with previous findings.41 However, the precise role of the 4G allele in obesity remains controversial since other authors have found a higher frequency of 4G/4G genotype in morbidly obese patients compared with a control group matched by age and sex.42 The prevalence of the 4G/4G genotype in the control group (27%), representing healthy Chilean subjects with BMI < 25 kg/m2, was similar to that reported in other series.37
When comparing the genotype of patients with liver fibrosis with those without fibrosis, no significant differences were observed in the genotype of the 4G/5G polymorphism of PAI-1 gene or in the allele distribution, showing that the population was in Hardy-Weinberg equilibrium. Moreover, we did not find differences in the genotype or allele distribution when we compared patients whom met histo-logical criteria of NASH associated with fibrosis, with patients without NASH.
PAI-1 serum levels were significantly lower in morbidly obese patients compared to control subjects. However, no differences were observed in morbidly obese patients with or without fibrosis. The sample size of this study was enough to demonstrate a significantly difference in PAI-1 serum levels between patients and controls but we will require 482 patients in the obese group and 673 subjects in the control group to demonstrate a difference in the 4G/ 5G polymorphism, according to the allele frequency observed in both groups. A previous report by Ye-ner, et al.43 compared PAI-1 levels in patients with NASH and healthy subjects found significant differences in PAI-1 plasma levels, although liver fi-brosis status was not analyzed. Importantly, in our study, 92% of patients with fibrosis showed mild fibrosis (stage 1 mainly) and only 8% of the patients with had advanced fibrosis, therefore should be of interest to assess PAI-1 genotype in a larger group of patients with proven bridging fi-brosis or cirrhosis.
There is controversial information regarding hepatic steatosis, in Argentinean population the 4G/ 5G PAI-1 polymorphism is not associated with the presence of obesity,17 but in subjects with NAFLD there is a higher serum levels of PAI-1 as well as in PAI-1 gene expression in liver biopsies.8 These findings are conflicting with our data in which a trend to lower PAI-1 plasma levels was observed among patients with histological diagnosis of NA-FLD. Moreover, no significant differences were observed in PAI-1 serum levels among obese patients with and without liver fibrosis and > 300,000 patients in each group will be required to demonstrate a statistically significant difference considering the small difference observed in this study. The difference observed in the 4G allele (63% in patients with liver fibrosis and 65% in patients without liver fibrosis) also requires over 7,000 patients to be recruited in each group (7,355 and 11,768, respectively). The huge sample size required to establish a difference in PAI-1 plasma levels or 4G allele polymorphism among obese patients with or without liver fibrosis is showing an irrelevant role of PAI-1 in the pathophysiology of liver fibrosis in non-alcoholic fatty liver disease.
In summary, morbidly obese patients had significantly lower PAI-1 serum levels with similar PAI-1 4G/5G genotypes frequencies. The genotype frequencies of the 4G/5G polymorphism of PAI-1 gene in Chilean healthy population are similar to those described in other international series. In our population of morbidly obese patients we did not observe differences neither in the frequency of the 4G/5G polymorphism of the PAI-1 gene nor in PAI-1 serum levels. Finally, PAI-1 did not exhibit any relationship with presence of liver fibrosis in this patient series.
Abbreviations- •
PAI-1: Plasminogen activator inhibitor type 1.
- •
NAFLD: Non-alcoholic fatty liver disease.
- •
NASH: Non-alcoholic steatohepatitis.
- •
ECM: Extracellular matrix.
- •
NO: Nitric oxide.
- •
BMI: Body mass index.
- •
ALT: Alanine aminotransferase.
- •
AAI: Amerindian Admixture Index.
- •
HCV: Hepatitis C virus.
- •
HOMA-IR: Homeostasis model assessment-insulin resistance.
We appreciate the cooperation of the SAVAL Laboratory. We specially thank Lorena Azocar, Lore-na Letelier, Dannae Turiel and Luz Carreno for their valuable technical support.