Portal vein thrombosis (PVT) is one of the most common vascular disorders of the liver with significant morbidity and mortality. Large cohort studies have reported a global prevalence of 1%, but in some risk groups it can be up to 26%. Causes of PVT are cirrhosis, hepatobiliary malignancy, abdominal infectious or inflammatory diseases, and myeloproliferative disorders. Most patients with PVT have a general risk factor. The natural history of PVT results in portal hypertension leading to splenomegaly and the formation of portosystemic collateral blood vessels and esophageal, gastric, duodenal, and jejunal varices. Diagnosis of PVT is made by imaging, mainly Doppler ultrasonography. According to its time of development, localization, pathophysiology, and evolution, PVT should be classified in every patient. Some clinical features such as cirrhosis, hepatocellular carcinoma, and hepatic transplantation are areas of special interest and are discussed in this review. The goal of treatment of acute PVT is to reconstruct the blocked veins. Endoscopic variceal ligation is safe and highly effective in patients with variceal bleeding caused by chronic PVT. In conclusion, PVT is the most common cause of vascular disease of the liver and its prevalence has being increasing, especially among patients with an underlying liver disease. All patients should be investigated for thrombophilic conditions, and in those with cirrhosis, anticoagulation prophylaxis should be considered.
Vascular diseases of the liver include a heterogeneous group of disorders resulting from hepatic, vascular, and cardiovascular causes. The term “portal vein thrombosis” (PVT) refers to an obstruction in the trunk of the hepatic portal vein. It is important to describe the anatomy of the portal vein to understand how the thrombosis or its obstruction might occur. The portal vein accounts for 75% of the blood supply to the liver. It is an 8-cm wide, valveless conduit originating from the confluence of the superior mesenteric and splenic veins posterior to the neck of the pancreas.
The importance of this vascular disease of the liver lies in its significant morbidity and mortality, which can occur without timely diagnosis or disease-specific management, or even with an inappropriate work-up.1 Its clinical presentation, prognosis, and management vary substantially according to etiology. For these reasons the cause must be investigated for each patient. In this review, we will discuss only PVT because most of the other vascular disorders of the liver are so rare (Table 1).
Vascular diseases of the liver.
| Budd-Chiari syndrome |
| Sinusoidal obstruction syndrome (veno-occlusive disease) |
| Portal vein thrombosis (PVT) |
| Ischemic hepatitis |
| Congestive hepatopathy |
| Peliosis hepatis |
| Hepatic artery aneurysm |
| Hepatic artery atherosclerosis |
| Congenital vascular malformations |
| Radiation-induced liver disease |
The concept of PVT as a rare disease is mainly based on clinical series and case reports. Estimates of the frequency and distribution of etiology vary widely between studies. In a large cohort of Sweden autopsies by Ögren, et al., a population prevalence of 1% was found.2 The reported prevalence of nontumorous PVT in patients with cirrhosis is highly variable at 0.6-26%, probably because of the different groups of affected patients studied and the different diagnostic procedures used. The incidence of de novo thrombosis within one year was reported as 16% by Amitrano, et al. in a group of patients with cirrhosis followed up prospectively during a similar time.3
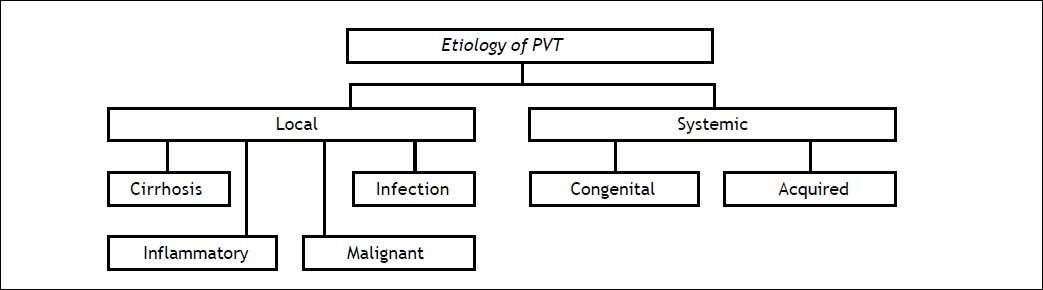
EtiologyIn a Swedish study of 254 autopsies, 28% had cirrhosis, 23% a primary hepatobiliary malignancy, 44% secondary hepatobiliary malignancy, 10% abdominal infectious of inflammatory disease, and up to 3% a myeloproliferative disorder2 (Figure 1). It has been reported that patients with cirrhosis and hepatic carcinoma have a high risk of PVT (odd ratio, OR, 17.1; 95% confidence interval, CI, 11.1-26.4). Is also important to note that the cause was not found in 14% of the patients.2,4
Risk factorsPVT is caused by a combination of local and general risk factors. Local risk factors can be identified in about 30% of patients, and a general risk factor in 70%. Local factors include cancers (any abdominal organ); focal inflammatory lesions (diverticulitis, appendicitis, pancreatitis, duodenal ulcer, cholecystitis, Crohn’s disease, or cytomegalovirus hepatitis); injury to the portal venous system (splenectomy, colectomy, gastrectomy, cholecystectomy, liver transplantation, or abdominal trauma); and cirrhosis with preserved liver function with precipitating factors, or advanced disease without an obvious precipitating factor. General risk factors for PVT include myeloproliferative disorders (40%), factor V Leiden mutation (32%), factor II mutation (40%), proteins C and S (26 and 30%), recent pregnancy (40%), antiphospholipid syndrome (19%), recent contraceptive use (12%), hyperhomocysteinemia (22%), and paroxysmal nocturnal hemoglobinuria (2%), among others.1
GeneticsCurrently it is known that genetic mutations are important underlying factors that increase the predisposition to venous thromboses and thromboembolisms. In the next paragraphs we will discuss briefly some of the main mutations.
Factor V (FV) is a protein involved in blood coagulation that acts as a cofactor in transforming prothrombin into thrombin, leading to fibrin formation. The C1691G>A mutation results in arginine at position 506 being replaced with glutamine, leading to the occurrence of Factor V Leiden (FVL), which increases the tendency to thrombosis.5
A G20210A mutation in the prothrombin gene (PTM) and the increase in mRNA production caused by transitions between guanine and adenine nucleotides at the 20210 position lead to an increase in prothrombin level and in the risk of thromboembolic disease.6
Methylene tetrahydrofolate reductase (MTHFR) plays a role as a transmethylation enzyme catalyzing methionine synthesis in DNA. Therefore mutations to the gene encoding MTHFR reduce methylation synthesis in DNA and cause hypercoagulation, which progresses together with plasma homocysteine volume. Polymorphisms in this gene are found in regions C677T and A1298C.6
Plasminogen Activator Inhibitor-Type 1 (PAI-1) is a specific plasminogen activator inhibitor that is released from endothelial cells, hepatocytes, and megakaryocytes. The PAI-1 plasma level increases in response to mutations in its encoding gene, and as this inhibits the fibrinolysis pathway, an increase in coagulation can be observed.6
Finally, Simsek, et al. have proposed that combined mutations above mentioned rather than single mutations, play key roles in causing thromboses and thromboembolisms.6
Natural historyPortal vein obstruction results from thrombosis, constriction, or invasion of the lumen. The resulting portal hypertension leads to splenomegaly and formation of portosystemic collateral vessels, and esophageal, gastric, duodenal, and jejunal varices. In the porta hepatis, varices proliferate and involve the gallbladder and bile duct. As the portal vein thromboses evolve, fibroblasts transform the clot into a firm, collagenous plug in which tortuous venous channels develop. This cavernous transformation (portal cavernoma) begins within days of the acute thrombosis and continues to evolve over weeks to months. Upstream from the obstruction, the small intestine and colon become congested, and the stomach exhibits changes of portal hypertensive gastropathy. Mesenteric ischemia can occur if the thrombus extends into the mesenteric veins.
ClassificationAccording to the time of development, localization, pathophysiology, and evolution, PVT can be classified as follows:
- •
Acute or chronic.
- •
Extra-or intrahepatic.
- •
Occlusive or nonocclusive.
- •
Progressive or self-resolving.
Acute PVT is characterized by the sudden formation of a thrombus within the portal vein. This can involve a variable portion of the mesenteric veins and/or the splenic vein. Occlusion can be complete or it can be partial, leaving a peripheral circulating channel. Acute PVT has been reported only rarely in children and is characterized by the presence of infected thrombus/thrombi.1
Chronic PVT, also known as a portal cavernoma, is described as such because the obstructed portal vein is replaced by a network of hepatopetal collateral veins connecting the patent portion of the vein upstream from the thrombus to the patent portion downstream. The number, size, and location of collaterals are extremely variable from patient to patient. With occlusion of the trunk of the portal vein, the antral, duodenal, and biliary veins are enlarged markedly. This enlargement can produce compression and deformation of the large bile ducts: socalled portal cholangiopathy or portal biliopathy. With occlusion at the origin of the portal vein, the pancreatic veins are enlarged.1
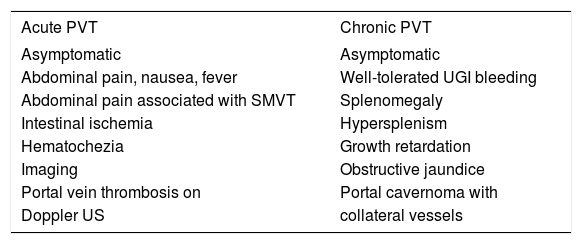
Clinical presentationApproximately 43% of patients with PVT can be asymptomatic and diagnosis can be done during a routine Doppler ultrasound (US) examination; 39% of the patients present with gastrointestinal bleeding and 18% present with acute abdominal pain. Abdominal pain arises when the thrombosis is acute or involves the mesenteric veins and causes intestinal ischemia. Splenomegaly is usually present, but ascites is uncommon, except in acute PVT or when the thrombosis complicates cirrhosis. Liver biochemical test results are usually normal.
In a univariate analysis published by Amitrano, et al7age, gender, previous endoscopic therapy of esophageal varices, previous abdominal surgery, and the Child-Pugh score did not influence the clinical presentation of PVT, and the site of the thrombosis did not affect this except for involvement of the mesenteric vein. In addition, the site of the PVT (portal trunk, intra-hepatic branches, or splenic) and type (occluding or partial) did not differ among asymptomatic and hemorrhagic patient (Table 2).7
Clinical presentation of PVT.17
| Acute PVT | Chronic PVT |
|---|---|
| Asymptomatic | Asymptomatic |
| Abdominal pain, nausea, fever | Well-tolerated UGI bleeding |
| Abdominal pain associated with SMVT | Splenomegaly |
| Intestinal ischemia | Hypersplenism |
| Hematochezia | Growth retardation |
| Imaging | Obstructive jaundice |
| Portal vein thrombosis on | Portal cavernoma with |
| Doppler US | collateral vessels |
SMVT: superior mesenteric vein thrombosis. US: ultrasonography. UGI: upper gastrointestinal.
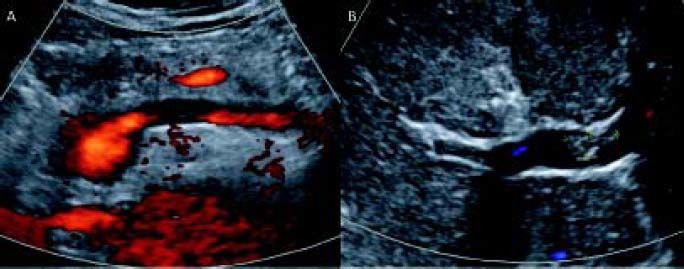
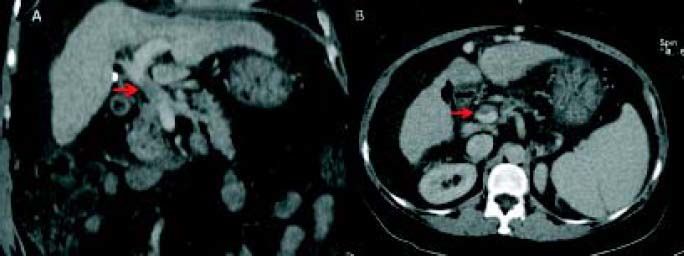
Plain and color Doppler US are almost always sufficient for diagnosis. Contrast-enhanced US was shown to be superior to plain US and color Doppler US for the detection and characterization of PVT (Figure 2). The gold standard of invasive angiography such as portal venography or superior mesenteric arteriography is rarely necessary. Retrograde carbon-dioxide portography can make PVT more evident than conventional computed tomography (CT) or magnetic resonance imaging (MRI), especially when an important hepatofugal flow is present. However, CT and MRI are better for determining the extent of thrombosis (Figure 3). Contrast-enhanced US or CT imaging can help differentiate cases of benign from malignant PVT. After diagnosis, further evaluation with upper gastrointestinal endoscopy is warranted to assess the presence and degree of esophageal varices. As current imaging techniques allow the detection of asymptomatic PVT during routine US examinations, increasing numbers of patients with cirrhosis are being diagnosed with PVT.4
PVT is usually an incidental radiological finding in patients with cirrhosis. This is especially true for partial PVT, where it is expected that portal flow will not be affected dramatically by the development of thromboses. In fact, in a significant proportion of patients (up to 50%) found to have PVT at the time of transplant surgery, the condition was unrecognized previously.8,9
In cases of acute PVT, US can show hyperechoic material in the vessel lumen with distension of the portal vein and its tributaries. Doppler US imaging shows the absence of blood flow in part or all of the lumen. CT scans without contrast can show hyper-attenuating material in the portal vein. After injection of a contrast agent, there is lack of luminal enhancement, increased hepatic enhancement in the arterial phase, and decreased hepatic enhancement in the portal phase. For assessing thrombus extension within the portal venous system, CT or MRI-based angiography are more sensitive techniques than color Doppler ultrasonography, because the mesenteric veins are more difficult to visualize with the latter technique. Some recommend that careful screening for PVT is important for all patients with cirrhosis and for those being evaluated for liver transplantation.10
Chronic PVT is diagnosed by documenting a cavernoma, with abdominal US, CT, or MRI showing serpiginous structures while the main portal vein and/or its main branches are not visible. The hepatic arteries are usually enlarged. In the absence of cirrhosis, there might be an enlarged caudate lobe, together with an atrophic left lateral segment or right lobe of the liver. Typically, the umbilical vein is not dilated as it connects to the left portal vein branch downstream of the obstruction.1
PVT and cirrhosisThe development of PVT is a significant milestone in the natural history of cirrhosis; it is associated with worsening liver function, ascites, and the occurrence of gastroesophageal variceal bleeding. The causal association between cirrhosis and PVT has been the subject of investigations but studies have not been able to address this association, or evaluate whether the development of PVT is just a consequence of advanced liver disease. The development of PVT was found to be more frequent in patients with advanced liver disease, but a low portal blood flow velocity was the only factor independently predicting the occurrence of PVT.11 A portal blood flow velocity < 15 cm/s at initial Doppler US evaluation was associated with a higher incidence of PVT (47.8%) than a portal blood flow velocity > 15 cm/s (2%).12
Traditionally, cirrhosis has been considered a hypocoagulable state, and the degree of prolongation of prothrombin time (PT) and international normalized ratio (INR) have been taken as markers of the severity of coagulopathy. We should remember that these values were designed primarily to assess hypo-coagulability in patients being treated with vitamin K antagonists, but in patients with liver disease they probably overestimate the bleeding risk. This might explain the paradox of the poor prediction of bleeding in cirrhotic patients, even with marked prolongation of conventional coagulation tests. It appears that in the setting of hepatic synthetic impairment, both pro- and anticoagulant proteins are reduced to a similar degree and the net result in most patients with cirrhosis is a compensated hemostatic balance with no tendency for bleeding or thrombosis. Because all of the components in the extrinsic coagulation pathway are produced by hepatocytes, the degree of prolongation of the PT has been used extensively as a measure of liver synthetic function. However, even anticoagulants such as Proteins C and S as well as the levels of circulating protease inhibitors are reduced in cases of hepatic insufficiency, favoring a hypercoagulable environment10 (Table 3).
A recent French multicenter prospective randomized trial carried out in patients with Child-Pugh types A and B cirrhosis who were subjected to Doppler US for screening of hepatocellular carcinoma (HCC) to identify risk factors for, and the impact of the development of, PVT. The investigators found that PVT was not a direct consequence of the progression of liver disease. Furthermore, no evidence of a direct impact of the development of PVT on the progression of liver disease was found.13
PVT and HCCPVT is a common complication of HCC with a reported incidence of 34% to 50%. It is one of the most negative prognostic factors and is called portal vein tumor thrombosis (PVTT). Survival in patients with PVTT is heterogeneous, depending on other clinical characteristics and hepatic function. Patient survival was reported to be less than 3 months in the absence of any treatment; however it has been shown to vary widely, from less than 5 months to more than 5 years depending on patient and tumor characteristics.
Previous staging systems of HCC did not take into account these characteristics, but more recent staging systems, such as the Barcelona Clinic Liver Cancer (BCLC) grading system, classify all patients with vascular/portal invasion and or extrahepatic spread as having HCC stage C, and it seems that Sorafenib is the only recommended treatment. It has been suggested that vascular endothelial growth factor plays a pivotal role in angiogenesis in patients with HCC and in the onset and evolution of PVTT, and that Sorafenib could exert a beneficial effect on PVTT by inhibition of the vascular endothelial growth factor receptor pathway with an antithrombotic effect and revascularization.14 Other locoregional therapies such as transarterial chemoembolization, radioembolization, and radiotherapy an also be considered because in theory these can suppress PVTT progression, delay intravascular tumor growth, and prevent the deterioration of liver function by maintaining an adequate portal blood flow.15
PVT and liver transplantation
PVT is a well-recognized complication in patients with liver cirrhosis waiting for liver transplantation (LT), and might recur after surgery. Some studies have reported a prevalence of 7.9% of PVT in this group of patients, but it can be as high as 26%.16 An accurate screening for PVT with determination of its grade of extension is mandatory, and should routinely be performed in the preoperative work-up of candidates for LT.
Historically, PVT was a contraindication for LT, but in recent years the indication of LT in these pa-
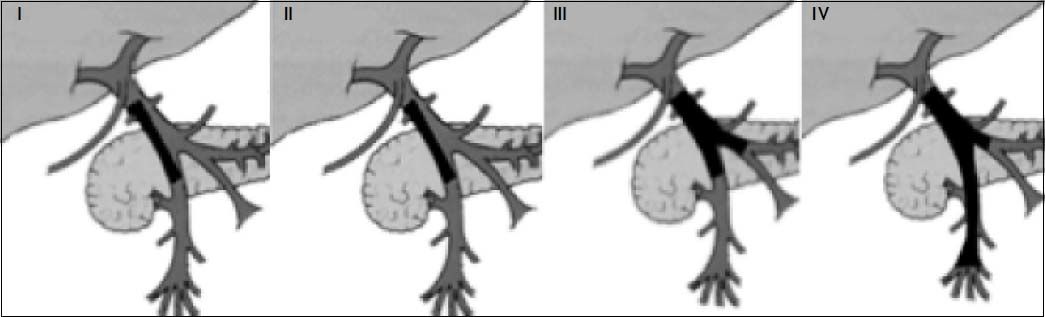
tients depends on the extent of the thrombosis. Early grades (I and II) do not contraindicate transplantation, but a questionable contraindication can be patients with grade III and IV PVT (Figure 4). There is no ideal surgical technique for performing LT in patients with PVT.
Stratification of PVT. Grade I: < 50% portal vein thrombosis, with or without minimal extension into the superior mesenteric vein. Grade II: > 50% occlusion of the portal vein, including total occlusions, with or without minimal extension into the superior mesenteric vein. Grade III: complete thrombosis of both portal and superior mesenteric veins; the distal superior mesenteric vein is patent. Grade IV: complete thrombosis of both portal vein and proximal and distal superior mesenteric veins.
The technical options for LT vary according to the patency of the portal vein and of other splanchnic veins. When full patency of the portal vein has been achieved, whether with anticoagulation or with a transjugular intrahepatic portosystemic shunt (TIPS), end-to-end portal anastomosis is the first step. When full patency has not been achieved, the technical option depends upon the extent of PVT (partial or complete) and the patency of splenic and mesenteric veins.17
Alterations in liver anatomy after LT also increases the intrahepatic resistance to portal flow, so endothelial injury and coagulopathies could lead to a prothrombotic tendency in these patients.18 Recurrence of PVT after LT is one of the most frequent postoperative complications, with a rate of 3-36%; however, depending on the series studied, the incidence of de novo PVT after LT in patients without any previous evidence is generally not specified.19
The main objective in the management of PVT in candidates for LT is to achieve at least partial recanalization so that portal flow to the graft can be restored through conventional end-to-end portal vein anastomosis. When recanalization is not achievable, the objective is to prevent any further extension of the thrombus during the waiting time, especially to the superior mesenteric vein.17
TreatmentThere is a growing need for optimal, evidence-based management of PVT in patients with cirrhosis because such management is currently not addressed in any consensus publication, including the recent practice guidelines on vascular disorders of the liver.4
The goal of treating acute PVT is to restore the obstructed veins, which will prevent intestinal infarction and portal hypertension. Correction of the causal factors should be achieved as soon as possible, but is beyond the scope of this review. If fever or leukocytosis is present, antibiotics can be used. There are reports of recanalization of pylephlebitis with antibiotic therapy alone.1
Early initiation of anticoagulation therapy-prefer-ably within 30 days of symptoms-is recommended because the rate of recanalization decreases from 69% to 25% when anticoagulation is instituted within the first or second week, respectively. Up to 35% of cases of acute PVT show recanalization with early anticoagulation therapy. Recanalization is less likely if the thrombosis is extensive because of the presence of more than one prothrombotic disorder, and is associated with ascites. Early anticoagulation therapy in patients with mesenteric and portal vein thromboses minimizes serious complications such as peritonitis because of bowel necrosis, and also significantly decreases the development of complications associated with esophageal varices. Recanalization occurs within 4-6 months after anticoagulation therapy.20 Long-term anticoagulation may be recommended in patients with identified prothrombotic disorders, recurrent episodes of thrombosis, or a family history of venous thrombosis. Anticoagulation can be initiated with heparin or subcutaneous low molecular weight heparin (for 2-3 weeks) because these are equally effective, although the second option makes laboratory monitoring unnecessary, with a lower risk of bleeding or immune thrombocytopenia. Later, oral vitamin K antagonists should be given to maintain an INR of 2-3. Thrombolytic therapy with recombinant tissue plasminogen activator, urokinase, and streptokinase in a very fresh PVT via a catheter introduced into the portal vein either transhepatically or through transjugular approach might improve regional clot lysis, with 75% achieving some degree of lysis of the thrombosis. However, there is a high rate of bleeding (60%) so thrombolytic therapy should be reserved for patients with severe disease.21
Varices appear as early as 1 month after PVT. The treatment of acute gastrointestinal bleeding caused by varices is similar to that used in patients with cirrhosis but without PVT. No studies have addressed the role of primary prophylaxis in PVT-associated portal hypertension. There is concern at the possible extension of thromboses with beta blockers as well as vasopressors because of a decrease in splanchnic blood flow.22 Endoscopic variceal ligation is safe and highly effective in children and adults with PVT. Variceal obliteration following endoscopic sclerotherapy opens up spontaneous shunts because of a possible increase in portal pressure in 40% of patients, which in turn protects these patients from further bleeding and recurrence of varices.23
Other therapeutic strategies include TIPS.24 Recent information suggests that TIPS should be considered a safe and feasible alternative therapy for selected patients with chronic PVT and cirrhosis, but it is not recommended for patients with a fibrotic cord and fine collaterals instead of the original portal vein. In addition, combining traditional TIPS with a transhepatic or trans-splenic approach may facilitate technical success. Successful TIPS effectively reduces the portosystemic pressure gradient and prevents the recurrence of variceal bleeding. Finally, the only predictor of survival in patients with PVT and cirrhosis might be the initial degree of occlusion, rather than any TIPS insertion.25
Some prophylactic strategies have been proposed, such as the use of enoxaparin to prevent PVT in patients with advanced cirrhosis awaiting LT, because in some randomized trials a 12-month course of enoxaparin was found to be safe and effective in preventing PVT in patients with cirrhosis and a Child-Pugh score of 7–10.26,27 Also, enoxaparin appeared to delay the occurrence of hepatic decompensation and to improve survival.28
ConclusionsOf all the vascular diseases of the liver, PVT is the most common and is being increasingly recognized with or without any underlying liver disease. All patients with PVT should be investigated for thrombophilic conditions as possible causes. Cirrhosis is considered the main course of PVT, and is a fluctuant disease. Therapy for hyper- and hypocoagulable conditions with low weight heparin should be considered in patients with PVT and cirrhosis, especially if waiting for a liver transplant. Imaging characteristics can help to distinguish patients with acute versus chronic PVT and will guide management of the patient.
AcknowledgementsThis project was support in part by a Grant from Medica Sur Clinic & Foundation.
Abbreviations- •
CT: computed tomography.
- •
FV: factor V.
- •
HCC: hepatocellular carcinoma.
- •
INR: international normalized ratio.
- •
LT: liver transplantation.
- •
MRI: magnetic resonance imaging.
- •
MTHFR: methylene tetrahydrofolate reductase.
- •
PAI-1: plasminogen Activator Inhibitor-Type 1.
- •
PT: prothrombin time.
- •
PVT: portal vein thrombosis.
- •
PVTT: portal vein tumor thrombosis.
- •
TIPS: transjugular intrahepatic portosystemic shunt.
- •
US: Doppler ultrasound.