We performed a systematic review and meta-analysis to evaluate the prevalence of concomitant Sjögren's syndrome (SS) with primary biliary cholangitis (PBC) in adults and quantify the impact of SS on PBC.
MethodsPubMed, Web of Science and Cochrane library were searched using subject terms and predefined inclusion and exclusion criteria.
ResultsSeventeen articles were included. The prevalence of SS in PBC patients ranged from 3.5 to 73% (35% pooled) (95% CI: 28–41%; p < 0.01). Seven studies included various biochemical indicators, including alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyltransferase (γ-GT), total bilirubin (TBiL), albumin (ALB) and platelet (PLT), and immunological indexes including IgG, IgM, antinuclear antibody (ANA), anti-mitochondrial antibody (AMA), AMA-M2 and anti-Ro/Sjögren's syndrome antigen A (SSA) antibodies. Meta-analysis showed that there were no significant differences in ALT, AST, ALP, γ-GT, TBiL and IgM levels between PBS and PBC with SS. Pooled analysis showed that ALB (MD=0.82; 95% CI: 0.08–1.56) and PLT (MD=30.41; 95% CI: 10.16–50.66) levels were lower, IgG levels (MD=-1.55; 95% CI: −2.39 to −0.72) were higher, and the positive ratios of ANA (RR=0.92; 95% CI: 0.87–0.98), AMA (RR=0.94; 95% CI: 0.89–0.98), AMA-M2 (RR=0.77; 95% CI: 0.70–0.85) and anti-Ro/SSA antibodies (RR=0.29; 95% CI: 0.08–1.01) were significantly higher in PBC patients with SS than in PBC patients.
ConclusionsOur study confirms that SS is common in PBC. Comorbid SS appears to influence the clinical phenotype of PBC and may therefore influence the management of PBC.
Primary biliary cholangitis (PBC) (formerly known as primary biliary cirrhosis) is a chronic autoimmune liver disease characterized by inflammatory destruction of intrahepatic interlobular bile ducts that leads to chronic cholestatic liver disease and eventually progresses to cirrhosis. Population-based studies reported that both the yearly incidence and prevalence of PBC seem to be increasing, and the incidence and prevalence of PBC have an uneven distribution around the world. The incidence of this disease is 1.7-54 cases per 100 000 people, while the prevalence is 18-492 cases per 100 000 people globally [1]. PBC has brought severe financial burdens to patients and these countries. The pathogenesis of PBC remains poorly understood. The causes of PBC appear to be a complex multistep process involving genetic and environmental factor interactions [2]. Furthermore, PBC may involve overlapping synthesis between autoimmune hepatology and systemic autoimmune disorders.
Sjögren's syndrome (SS) is a chronic, systemic autoimmune disease characterized by autoimmune epithelitis that leads to the destruction of exocrine glands, such as salivary glands and lacrimal glands, and commonly manifests as dry mouth and eyes. SS is divided into primary SS (pSS), which occurs alone, and secondary SS, which is usually associated with various other autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis and PBC [3, 4].
PBC is often associated with various systemic autoimmune diseases, such as thyroid disorders, systemic sclerosis (SSc) and SS. Several studies have indicated that the most common autoimmune disease related to PBC is SS; however, the prevalence of SS in PBC patients differs widely, ranging from 3.5% to 73% [5, 6]. Correspondingly, several studies have shown that the coexistence of SS and PBC highlights the different characteristics of clinical manifestation, laboratory data, and even prognosis compared to PBC alone. However, the characteristics remain a subject of debate.
Thus, the primary aim of this systematic review and meta-analysis is to evaluate the prevalence of SS in PBC in adult patients. The secondary aim is to compare index conditions between PBC with and without SS, and thereby assess the impact of comorbid SS on PBC.
2Materials and methods2.1Search strategyThis study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA). PubMed, Web of Science and Cochrane library were searched independently by two reviewers on 30 June 2021 using search terms: (“Primary biliary cholangitis” OR “primary biliary cirrhosis” OR “PBC”) AND (“Sjogren's syndrome” OR “Sjogren syndrome” OR “sicca syndrome” OR “SS”). No language restriction was placed.
2.2Inclusion and exclusion criteriaStudies met the following inclusion criteria if they: included patients of SS with a pre-existing diagnosis of PBC; stated the percentage of SS in PBC patients; evaluated laboratory parameters in PBC patients with or without SS; and were available in full text.
Studies were excluded if they: included patients of PBC with a pre-existing diagnosis of SS; included insufficient data; were irrelevant topics, reviews, case reports, letters, comments and non-human studies.
2.3Data extraction and quality assessmentThe relevant data were independently extracted by two reviewers into predefined tabulated summaries. Data included: first author, year, country; important characteristics of the patients (number, gender, age); the prevalence of SS with PBC; the biochemical and immunological characteristics in PBC patients with or without SS.
The methodological quality of each included study was assessed by two investigators using the Newcastle-Ottawa Scale (NOS). Studies with seven stars or more were deemed high quality.
2.4Statistical analysisA meta-analysis of prevalence was performed using R4.1.0 software (Wolfgang Viechtbauer). Prevalence estimates were presented as percentages. Meta-analysis of the biochemical and immunological characteristics was performed using Review Manager version 5.3 (RevMan, the Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
We calculated the mean differences (MD) and relative risk (RR) with corresponding 95% confidence intervals (95% CIs) for the meta-analysis to compare the levels of hematological parameters between patients with PBC and PBC complicating SS. The mean and standard deviation (SD) that reported the median and its interquartile range (IQR) in two studies was calculated using formulas [7].
The I2 statistic was used to assess the statistical heterogeneity of the meta-analysis. Random effects methods were used when statistical heterogeneity was high (I2>75%); otherwise, fixed effects models were selected when statistical heterogeneity was low or moderate (I2<75%). p < 0.05 was considered statistically significant [8]. Forest plots were produced in order to assess publication bias.
2.5Register name and registration numberCRD42022346203
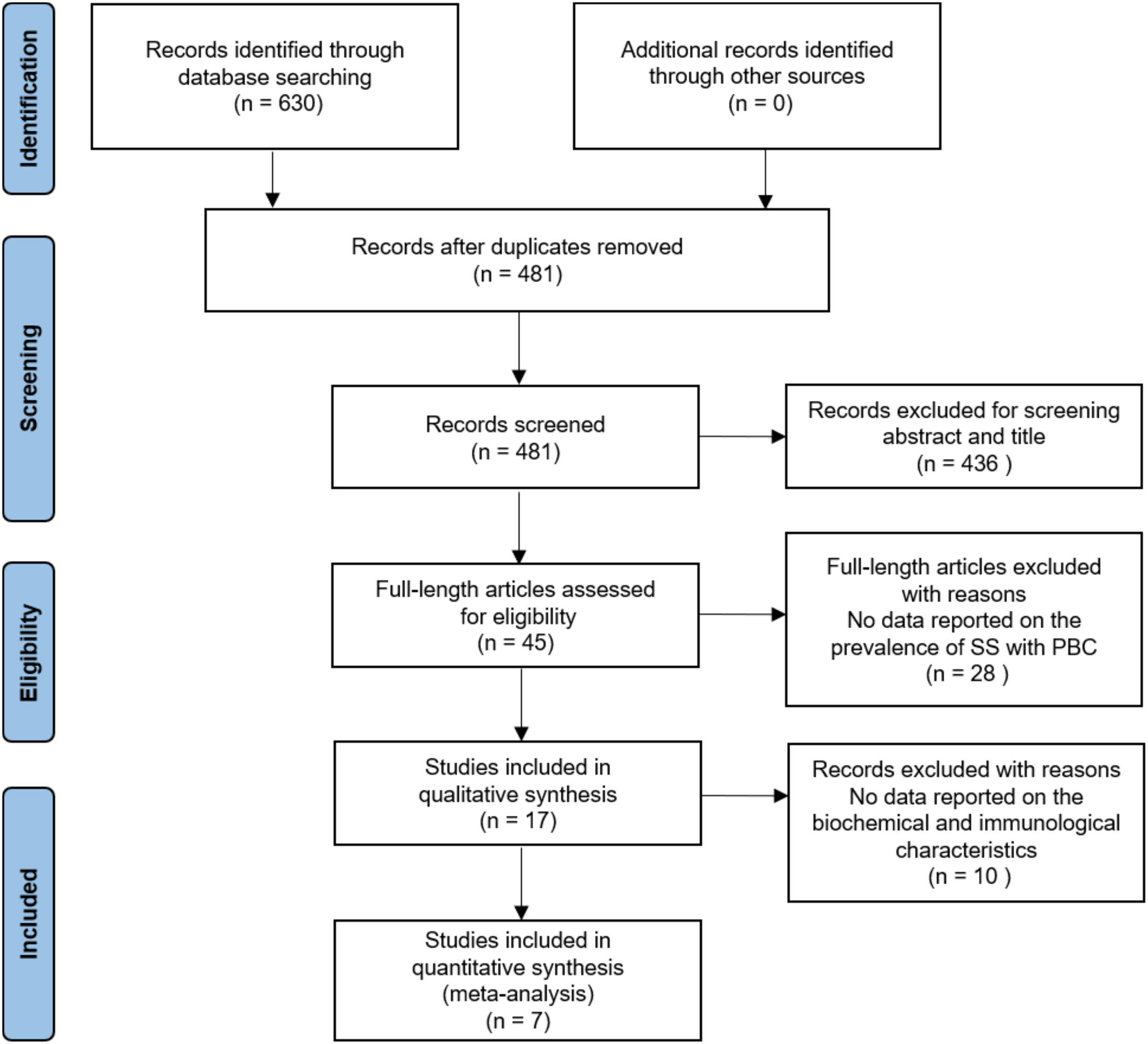
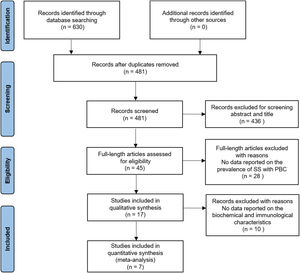
3Results3.1Literature search and study characteristicsA total of 630 relevant articles were identified through the database search. In total, 17 studies met the inclusion criteria in this systematic review and meta-analysis. Of the included studies, 17 studies reported data on the prevalence of SS in PBC [4–6, 9–22], and 7 studies presented laboratory data [4, 17–20]. The details of the PRISMA flowchart of literature search are provided in Fig. 1.
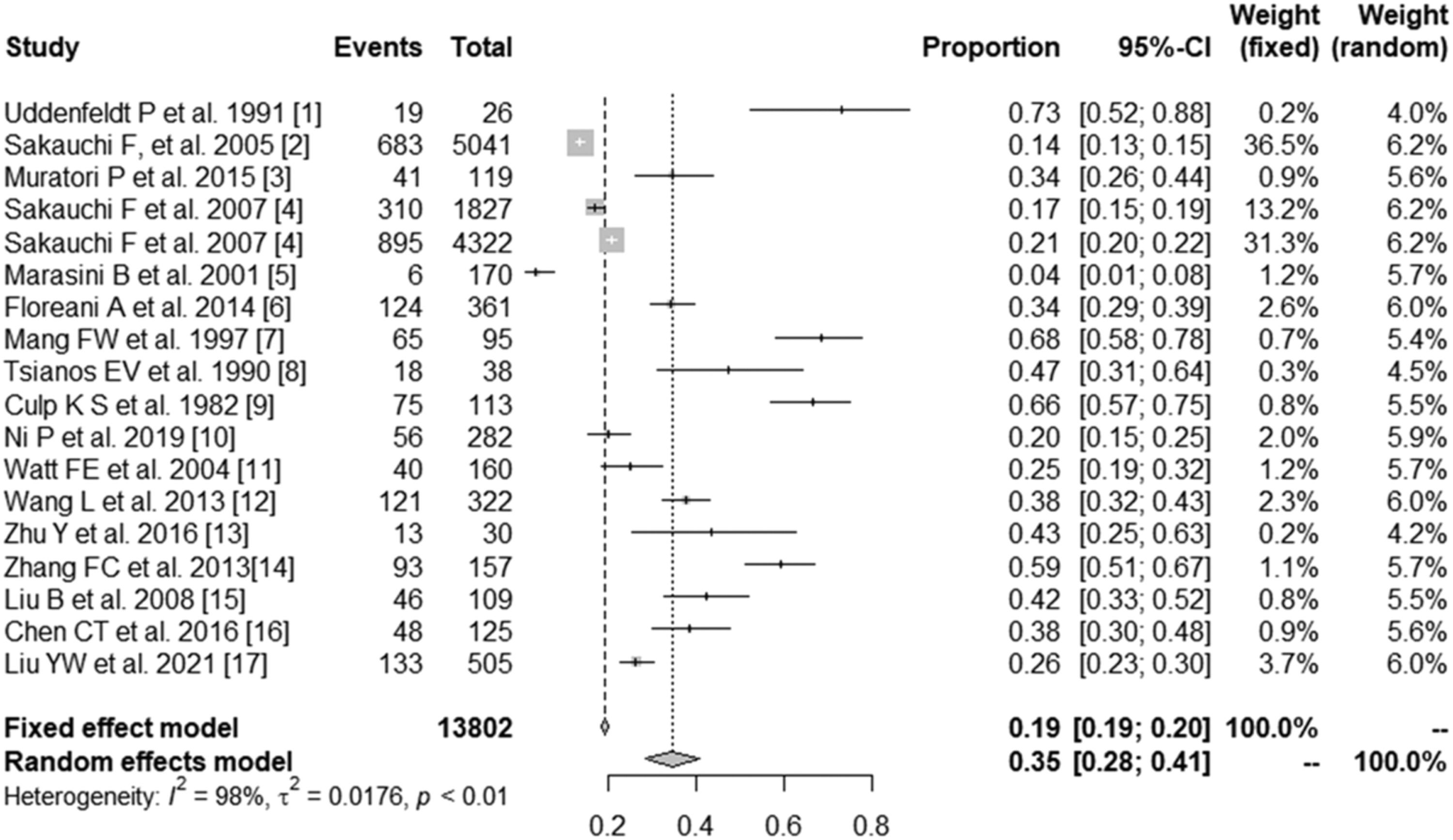
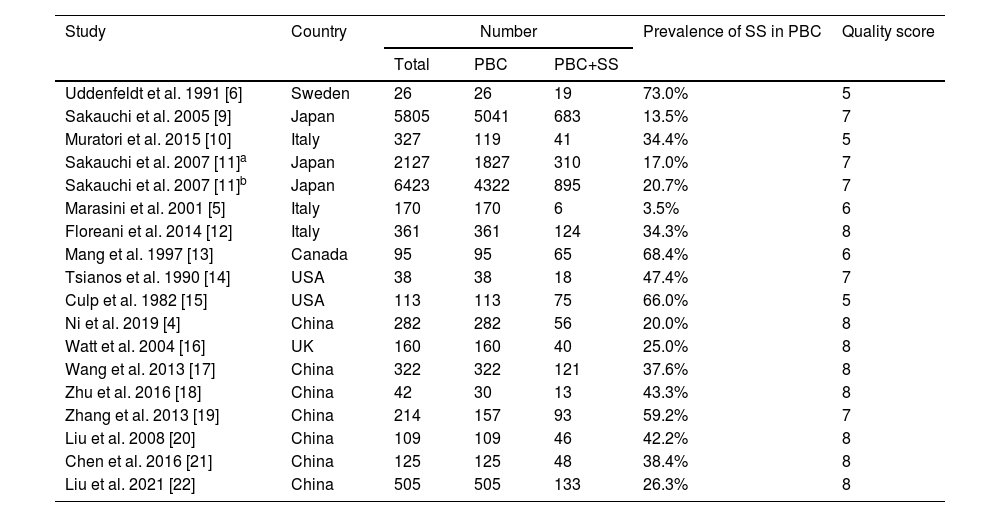
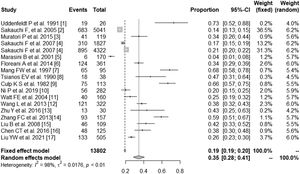
3.2Prevalence of SS in PBCSeventeen studies containing 13802 PBC patients reported the prevalence of SS, which ranged from 3.5% to 73%. In the proportional meta-analysis, the overall prevalence rate of PBC with SS was 35% (95% CI: 28%–41%; p < 0.01) across all studies. Heterogeneity was high between these studies for meta-analysis of prevalence (I2=98%), and the random model was selected (Fig. 2). Funnel plots showed the existence of publication bias (Supplementary Fig. S1). The studies with their characteristics are outlined in Table 1.
The characteristics of included studies in the meta-analysis.
| Study | Country | Number | Prevalence of SS in PBC | Quality score | ||
|---|---|---|---|---|---|---|
| Total | PBC | PBC+SS | ||||
| Uddenfeldt et al. 1991 [6] | Sweden | 26 | 26 | 19 | 73.0% | 5 |
| Sakauchi et al. 2005 [9] | Japan | 5805 | 5041 | 683 | 13.5% | 7 |
| Muratori et al. 2015 [10] | Italy | 327 | 119 | 41 | 34.4% | 5 |
| Sakauchi et al. 2007 [11]a | Japan | 2127 | 1827 | 310 | 17.0% | 7 |
| Sakauchi et al. 2007 [11]b | Japan | 6423 | 4322 | 895 | 20.7% | 7 |
| Marasini et al. 2001 [5] | Italy | 170 | 170 | 6 | 3.5% | 6 |
| Floreani et al. 2014 [12] | Italy | 361 | 361 | 124 | 34.3% | 8 |
| Mang et al. 1997 [13] | Canada | 95 | 95 | 65 | 68.4% | 6 |
| Tsianos et al. 1990 [14] | USA | 38 | 38 | 18 | 47.4% | 7 |
| Culp et al. 1982 [15] | USA | 113 | 113 | 75 | 66.0% | 5 |
| Ni et al. 2019 [4] | China | 282 | 282 | 56 | 20.0% | 8 |
| Watt et al. 2004 [16] | UK | 160 | 160 | 40 | 25.0% | 8 |
| Wang et al. 2013 [17] | China | 322 | 322 | 121 | 37.6% | 8 |
| Zhu et al. 2016 [18] | China | 42 | 30 | 13 | 43.3% | 8 |
| Zhang et al. 2013 [19] | China | 214 | 157 | 93 | 59.2% | 7 |
| Liu et al. 2008 [20] | China | 109 | 109 | 46 | 42.2% | 8 |
| Chen et al. 2016 [21] | China | 125 | 125 | 48 | 38.4% | 8 |
| Liu et al. 2021 [22] | China | 505 | 505 | 133 | 26.3% | 8 |
a and b, two different clinical data in the two different years (1999 and 2004) from the same article.
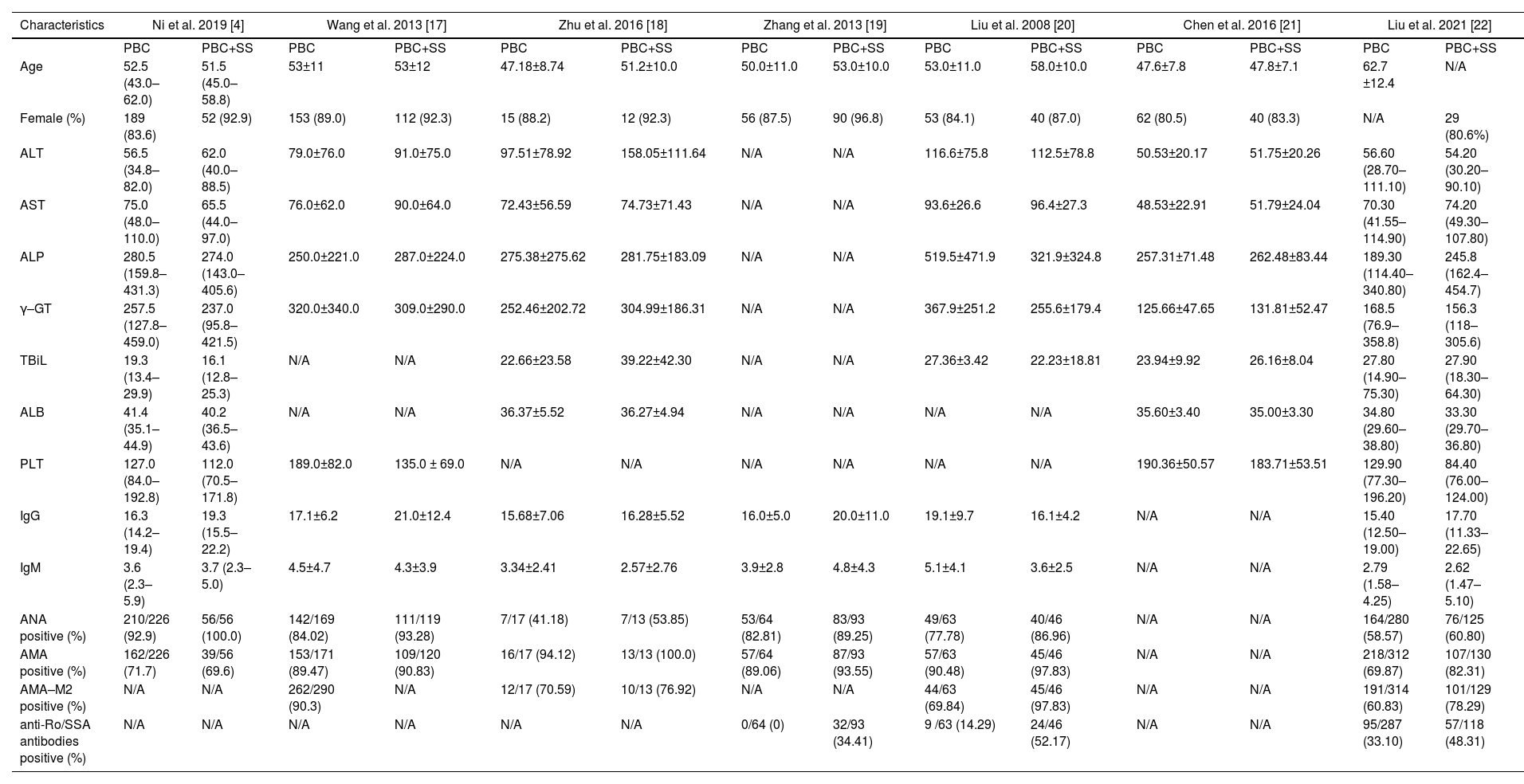
Of the included studies, seven articles reported the biochemical and immunological characteristics in PBC patients with or without SS. In terms of different biochemical and immunological indexes, we performed thirteen subgroup meta-analyses, including ALT, AST, ALP, γ-GT, TBiL, ALB, PLT, IgG, IgM, ANA, AMA, AMA-M2, and anti-Ro/SSA antibodies. The characteristics of the studies and clinical parameters are listed in Table 2.
Main data of included studies in the meta-analysis.
| Characteristics | Ni et al. 2019 [4] | Wang et al. 2013 [17] | Zhu et al. 2016 [18] | Zhang et al. 2013 [19] | Liu et al. 2008 [20] | Chen et al. 2016 [21] | Liu et al. 2021 [22] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBC | PBC+SS | PBC | PBC+SS | PBC | PBC+SS | PBC | PBC+SS | PBC | PBC+SS | PBC | PBC+SS | PBC | PBC+SS | |
| Age | 52.5 (43.0–62.0) | 51.5 (45.0–58.8) | 53±11 | 53±12 | 47.18±8.74 | 51.2±10.0 | 50.0±11.0 | 53.0±10.0 | 53.0±11.0 | 58.0±10.0 | 47.6±7.8 | 47.8±7.1 | 62.7 ±12.4 | N/A |
| Female (%) | 189 (83.6) | 52 (92.9) | 153 (89.0) | 112 (92.3) | 15 (88.2) | 12 (92.3) | 56 (87.5) | 90 (96.8) | 53 (84.1) | 40 (87.0) | 62 (80.5) | 40 (83.3) | N/A | 29 (80.6%) |
| ALT | 56.5 (34.8–82.0) | 62.0 (40.0–88.5) | 79.0±76.0 | 91.0±75.0 | 97.51±78.92 | 158.05±111.64 | N/A | N/A | 116.6±75.8 | 112.5±78.8 | 50.53±20.17 | 51.75±20.26 | 56.60 (28.70–111.10) | 54.20 (30.20–90.10) |
| AST | 75.0 (48.0–110.0) | 65.5 (44.0–97.0) | 76.0±62.0 | 90.0±64.0 | 72.43±56.59 | 74.73±71.43 | N/A | N/A | 93.6±26.6 | 96.4±27.3 | 48.53±22.91 | 51.79±24.04 | 70.30 (41.55–114.90) | 74.20 (49.30–107.80) |
| ALP | 280.5 (159.8–431.3) | 274.0 (143.0–405.6) | 250.0±221.0 | 287.0±224.0 | 275.38±275.62 | 281.75±183.09 | N/A | N/A | 519.5±471.9 | 321.9±324.8 | 257.31±71.48 | 262.48±83.44 | 189.30 (114.40–340.80) | 245.8 (162.4–454.7) |
| γ–GT | 257.5 (127.8–459.0) | 237.0 (95.8–421.5) | 320.0±340.0 | 309.0±290.0 | 252.46±202.72 | 304.99±186.31 | N/A | N/A | 367.9±251.2 | 255.6±179.4 | 125.66±47.65 | 131.81±52.47 | 168.5 (76.9–358.8) | 156.3 (118–305.6) |
| TBiL | 19.3 (13.4–29.9) | 16.1 (12.8–25.3) | N/A | N/A | 22.66±23.58 | 39.22±42.30 | N/A | N/A | 27.36±3.42 | 22.23±18.81 | 23.94±9.92 | 26.16±8.04 | 27.80 (14.90–75.30) | 27.90 (18.30–64.30) |
| ALB | 41.4 (35.1–44.9) | 40.2 (36.5–43.6) | N/A | N/A | 36.37±5.52 | 36.27±4.94 | N/A | N/A | N/A | N/A | 35.60±3.40 | 35.00±3.30 | 34.80 (29.60–38.80) | 33.30 (29.70–36.80) |
| PLT | 127.0 (84.0–192.8) | 112.0 (70.5–171.8) | 189.0±82.0 | 135.0 ± 69.0 | N/A | N/A | N/A | N/A | N/A | N/A | 190.36±50.57 | 183.71±53.51 | 129.90 (77.30–196.20) | 84.40 (76.00–124.00) |
| IgG | 16.3 (14.2–19.4) | 19.3 (15.5–22.2) | 17.1±6.2 | 21.0±12.4 | 15.68±7.06 | 16.28±5.52 | 16.0±5.0 | 20.0±11.0 | 19.1±9.7 | 16.1±4.2 | N/A | N/A | 15.40 (12.50–19.00) | 17.70 (11.33–22.65) |
| IgM | 3.6 (2.3–5.9) | 3.7 (2.3–5.0) | 4.5±4.7 | 4.3±3.9 | 3.34±2.41 | 2.57±2.76 | 3.9±2.8 | 4.8±4.3 | 5.1±4.1 | 3.6±2.5 | N/A | N/A | 2.79 (1.58–4.25) | 2.62 (1.47–5.10) |
| ANA positive (%) | 210/226 (92.9) | 56/56 (100.0) | 142/169 (84.02) | 111/119 (93.28) | 7/17 (41.18) | 7/13 (53.85) | 53/64 (82.81) | 83/93 (89.25) | 49/63 (77.78) | 40/46 (86.96) | N/A | N/A | 164/280 (58.57) | 76/125 (60.80) |
| AMA positive (%) | 162/226 (71.7) | 39/56 (69.6) | 153/171 (89.47) | 109/120 (90.83) | 16/17 (94.12) | 13/13 (100.0) | 57/64 (89.06) | 87/93 (93.55) | 57/63 (90.48) | 45/46 (97.83) | N/A | N/A | 218/312 (69.87) | 107/130 (82.31) |
| AMA–M2 positive (%) | N/A | N/A | 262/290 (90.3) | N/A | 12/17 (70.59) | 10/13 (76.92) | N/A | N/A | 44/63 (69.84) | 45/46 (97.83) | N/A | N/A | 191/314 (60.83) | 101/129 (78.29) |
| anti-Ro/SSA antibodies positive (%) | N/A | N/A | N/A | N/A | N/A | N/A | 0/64 (0) | 32/93 (34.41) | 9 /63 (14.29) | 24/46 (52.17) | N/A | N/A | 95/287 (33.10) | 57/118 (48.31) |
ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase, γ-GT, γ-glutamyltransferase; TBiL, total bilirubin; ALB, albumin; PLT, platelet; ANA, antinuclear antibody; AMA, anti-mitochondrial antibody; N/A, no available.
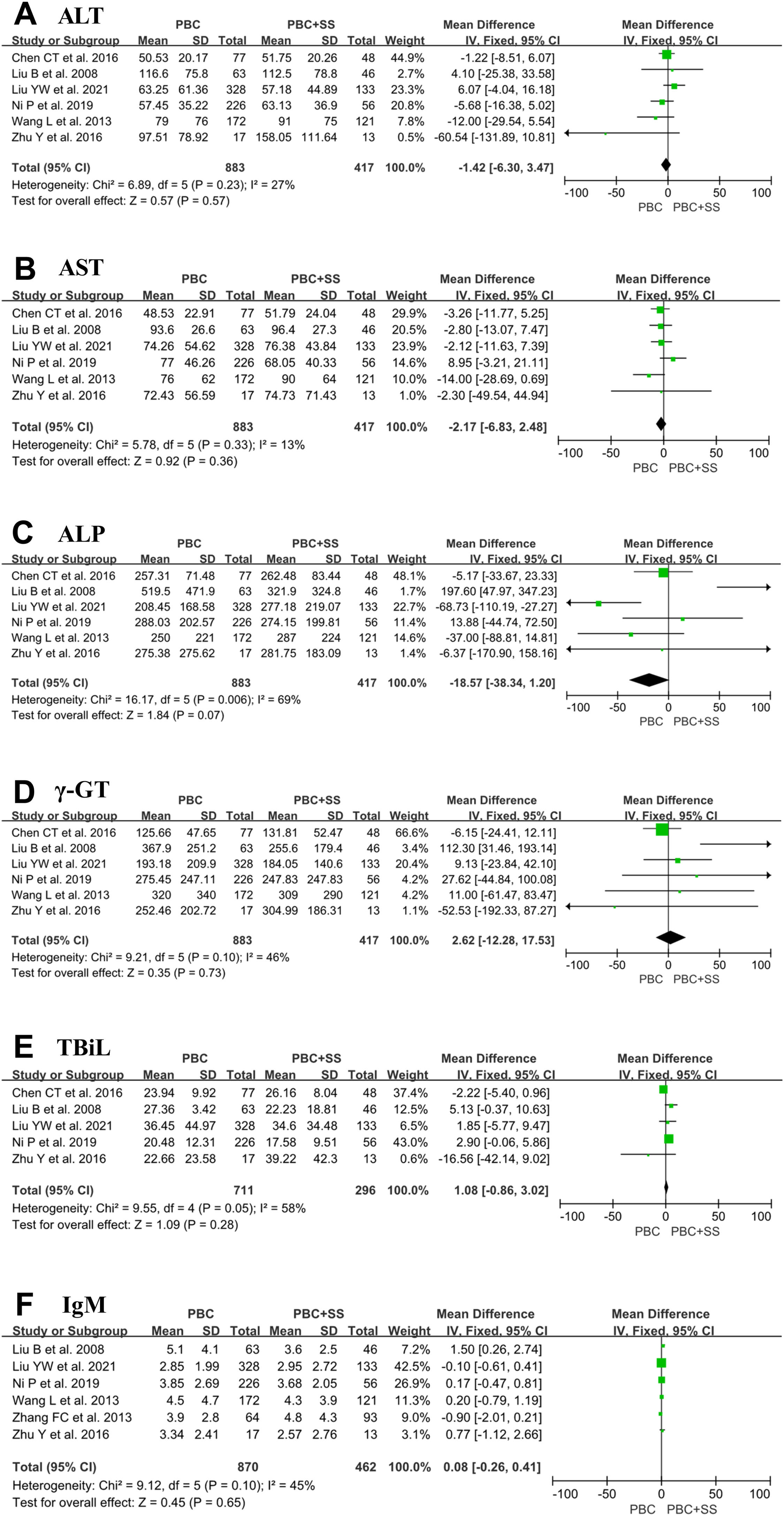
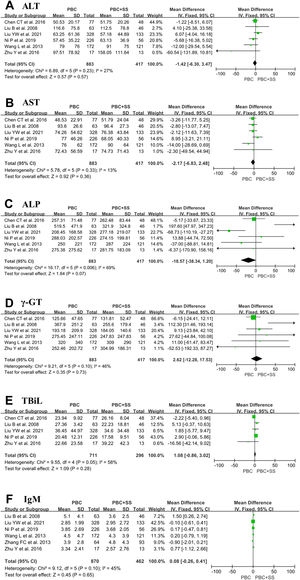
The results from six studies showed that there were no significant differences in ALT, AST, ALP, γ-GT and IgM levels between PBS and PBC with SS [4, 17, 18, 20–22]. Total pooled analysis results from five studies showed that there were no significant differences in TBiL levels between the two groups [4, 18, 20–22]. Other hematological indexes were significantly different between the PBC and PBC with SS groups (Fig. 3).
The forest plots of the biochemical and immunological indexes (ALT, AST, ALP, γ-GT, TBiL and IgM) for patients PBC with and without SS. (A) ALT; (B) AST; (C) ALP; (D) γ-GT; (E) TBiL; (F) IgM. CI, confidence interval; IV, inverse variance; SD, standard deviation; ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase, γ-GT, γ-glutamyltransferase; TBiL, total bilirubin.
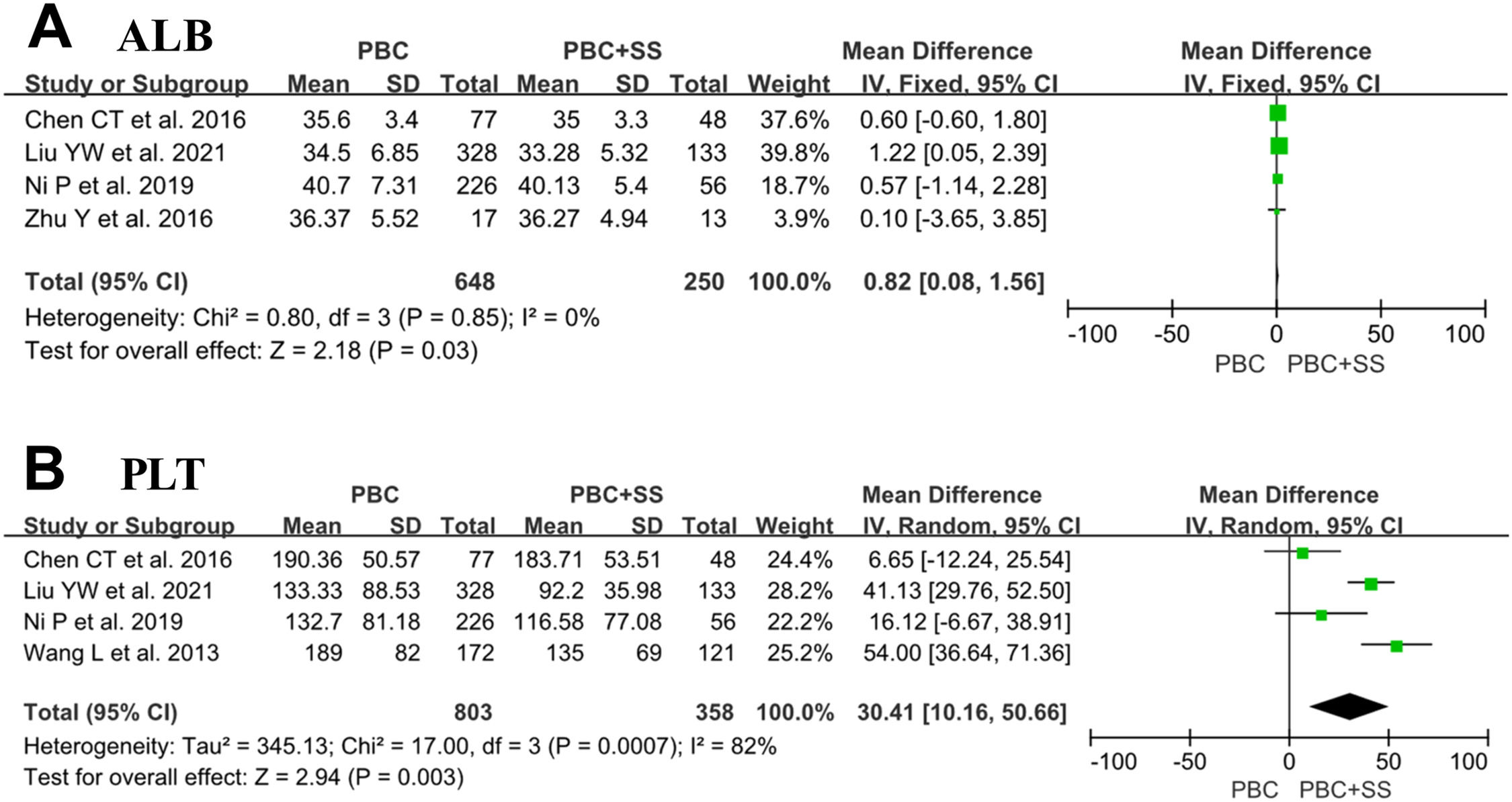
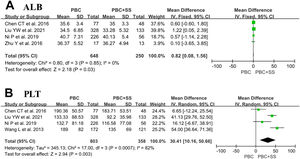
Four studies consisting of 648 PBC subjects and 250 PBC with SS subjects reported data on ALB [4, 18, 21, 22]. Our meta-analysis demonstrated significantly lower ALB in patients suffering from PBC with SS compared to PBC alone with MD of 0.82 (95% CI: 0.08–1.56; p = 0.03; I2=0%) (Fig. 4).
Four studies comprised 803 PBC patients and PBC with SS patients and approached the values of PLT [4, 18, 21, 22]. The analysis results showed that PBC patients with comorbid SS had significantly lower levels of PLTs than those with PBC alone (MD=30.41; 95% CI: 10.16–50.66; p = 0.003; I2=82%) (Fig. 4).
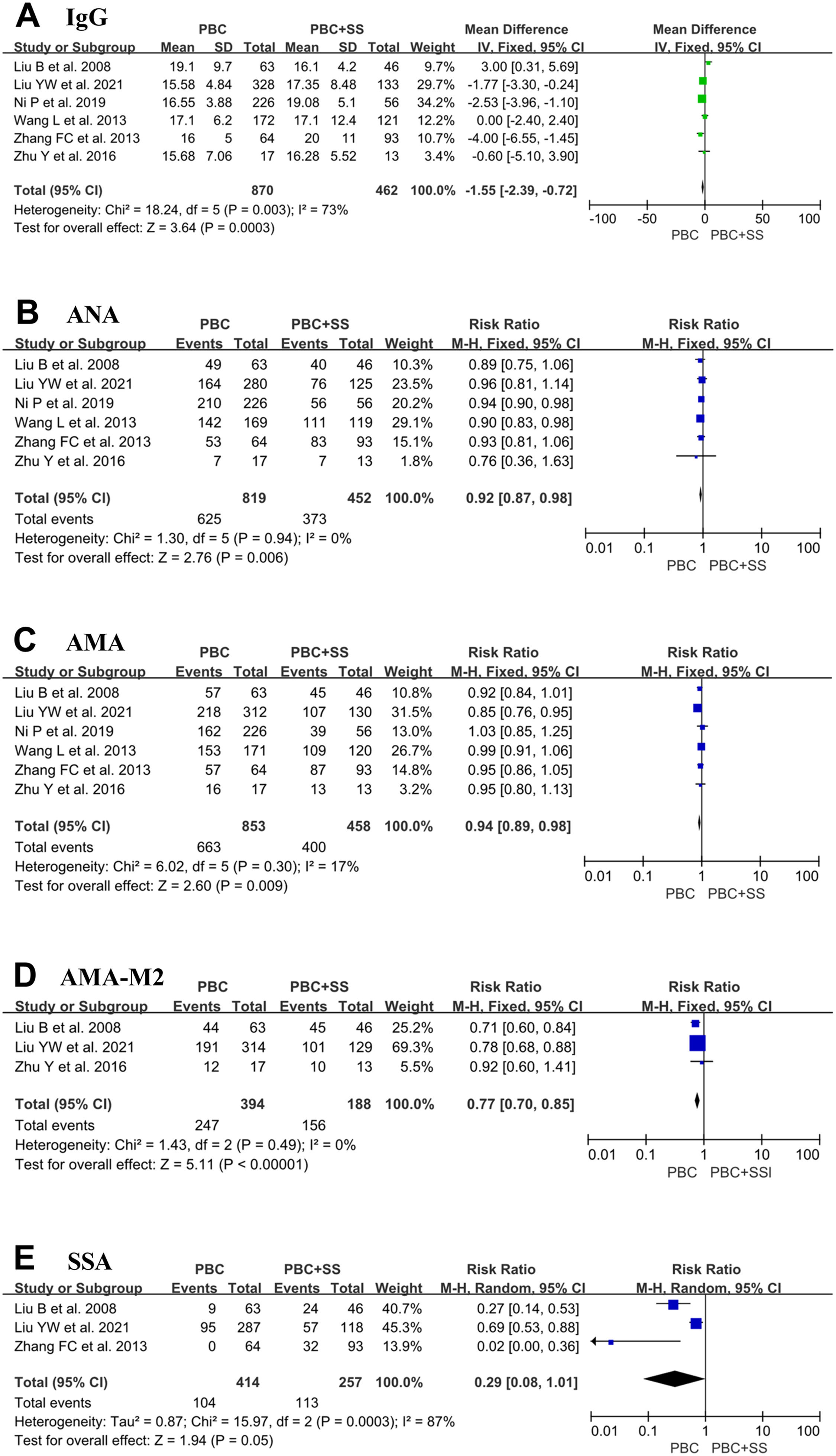
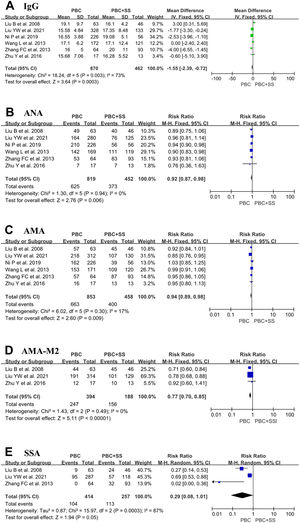
A total of 870 PBC patients and 462 PBC with SS patients were included from six studies, and the IgG values were evaluated [4, 17–20, 22]. The pooled result in PBC with SS demonstrated a significant increase in IgG compared with PBC alone (MD = -1.55; 95% CI: -2.39 to -0.72; p = 0.0003; I2=73%) (Fig. 5).
Forest plots of the immunological indexes (IgG, ANA, AMA, AMA-M2 and anti-Ro/SSA antibodies) for patients PBC with and without SS. (A) IgG; (B) ANA; (C) AMA; (D) AMA-M2; (E) anti-Ro/SSA antibodies. CI, confidence interval; IV, inverse variance; SD, standard deviation; ANA, antinuclear antibody; AMA, anti-mitochondrial antibody; SSA, Sjögren's syndrome antigen A.
Data from six studies comprised 819 patients with PBC alone and 452 patients with PBC with SS, which were pooled to compare the positive ratio of ANA [4, 17–20, 22]. Pooled analysis indicated that the positive ratio of ANA was higher in PBC patients with SS than in PBC patients (RR=0.92; 95% CI: 0.87–0.98; p = 0.006; I2=0%) (Fig. 5).
Six studies comprised 853 patients with PBC and 458 patients with PBS and SS reported the ratio of positive AMA [4, 17–20, 22]. Pooled analysis indicated that PBC patients with SS had a higher ratio of positive AMA than PBC patients, with RR 0.94 (95% CI: 0.89–0.98; p = 0.009; I2=17%) (Fig. 5).
Only three studies contained 394 PBC patients and 188 PBC with SS patients and reported the ratio of positive AMA-M2 [17, 18, 20, 22]. The overall pooled analyses showed that PBC patients with SS were associated with a higher ratio of positive AMA-M2 (RR=0.77; 95% CI: 0.70–0.85; p < 0.0001; I2=0%) (Fig. 5).
Only three studies contained consisting of 414 PBC subjects and 257 PBC with SS subjects reported a positive ratio of anti-Ro/SSA antibodies [19, 20, 22]. The total pooled analysis result showed that PBC patients with concomitant SS had a higher positive ratio of anti-Ro/SSA antibodies than PBC patients (RR=0.29; 95% CI: 0.08–1.01; p = 0.05; I2=87%) (Fig. 5).
3.4Evaluation of publication biasThe publication bias among the studies was assessed based on the funnel plot. All the results except the proportional meta-analysis indicated that there was no evidence of publication bias (Supplementary Fig. S1).
4DiscussionPBC is a liver tissue-specific disease characterized by chronic non-suppurative destructive cholangitis. However, PBC is also associated with other autoimmune diseases, including intrahepatic diseases, such as autoimmune hepatitis, and extrahepatic connective tissue diseases (CTDs), such as SS, SSs, and systemic lupus erythematosus (SLE). Current studies have shown that SS is one of the most common CTDs that coexist with PBC [18]. There is a difference in clinical symptoms, therapies and prognosis between patients with PBC alone and PBC accompanied by SS.
To the best of our knowledge, this is the first systematic review and meta-analysis to conclude the pooled prevalence of SS among PBC patients and analyze the difference in hematological parameters between the two groups. This present review found that concomitant SS is common in PBC. Our results showed that the overall prevalence of SS was 35% in PBC (range 3.5–73%). Heterogeneity was high in the meta-analysis of prevalence, and which suggesting that pooled results should be treated with caution. We stratified the meta-analysis by Asian/non-Asian region and study sample selection methods; however, there was no significant influence on heterogeneity by these factors (data not shown). The prevalence estimates are likely accounted for by factors including sample size, age and gender.
PBC presents with inflammatory lesions of intrahepatic small bile ducts but not hepatocyte destruction, so classification criteria for PBC include an elevated ALP and/or γ-GT level but not a change in ALT or AST. Although some studies revealed that SS might cause secretion impairment and inflammation in different organs involved in exocrine glands and other parenchymal organs, such as the lungs, pancreas and liver, some patients with SS have different degrees of elevation in the laboratory indexes, including ALT and AST levels. Of six studies included in this analysis, four studies and five studies suggested that ALT and AST levels were only slightly elevated, respectively [17, 18, 20–22]. Consistently, the results of our meta-analysis showed that both ALT and AST levels were not statistically significant between patients with PBC and PBC accompanied by SS. Similarly, of six studies included in the analysis, four studies indicated that a mildly higher level of ALP and γ-GT was detected in the PBC+SS group compared to the PBC group [17, 18, 21, 22]; however, our analyses showed that none were statistically significant in either ALP or γ-GT levels between the two groups. In addition, two studies discovered that ALP and γ-GT levels were reduced after ursodeoxycholic acid (UDCA) treatment for 12 months in both PBC alone and PBC+SS groups [4, 22]. These results appear to suggest that although SS may cause liver damage, SS does not aggravate hepatocyte and bile duct inflammation in PBC patients and does not affect the biochemical response of UDCA-treated patients with PBC.
In addition, of four studies included in the analysis, although all studies displayed lower ALB levels in the PBC+SS group than in the PBC group, only one study reached statistical significance [4, 18, 21, 22]. Surprisingly, our meta-analysis revealed that ALB levels showed a statistically significant difference between the two groups (p = 0.03). A Study by Liu reported that patients with PBC+SS had significantly elevated cirrhosis levels and a poor prognosis compared with patients having PBC [22]. Hence, patients with PBC+SS may have a longer course and advanced histological stage of PBC, leading to decreased serum albumin levels. Additionally, SS can affect gastrointestinal tract absorption function or cause intestinal inflammatory cell infiltration and ultimately result in protein loss in the intestinal lumen and hypoalbuminemia [23]. However, further studies will be needed to assess whether patients with PBC+SS who develop hypoalbuminemia have worse outcomes than patients with PBC alone. Furthermore, the results showed that PLT counts were lower in the PBC+SS group than in the PBC group (p = 0.003). The AST to PLT ratio index is usually used to evaluate the progression states of liver fibrosis; however, Chun-Ting Chen's study has reported that there were no significant differences in PLT counts, and the incidence of advanced liver fibrosis was also not significantly different between PBC patients and PBC+SS patients [21, 24]. Hence, the relationship between coexisting diseases and reduced blood PLT counts remains unclear and may send a signal of more extensive liver damage in the coexistence of SS in PBC patients.
Next, we reviewed the difference in immunological indexes between PBC alone and comorbid SS in PBC. Our results revealed that patients in the PBC+SS group had the higher positive rates of ANA (p = 0.006), AMA (p = 0.009), AMA-M2 (p < 0.0001), and anti-Ro/SSA antibodies (p = 0.05), and significantly elevated levels of serum IgG (p < 0.0001) than those with PBC. However, although 1 study showed that the serum IgM levels of PBC patients without SS were higher than those of patients with SS [20], our analysis suggested that the serum IgM levels in PBC patients without SS were not significantly different from those in PBC patients with SS. Serum ANA is the most common non-organ-specific autoantibody, with positive rates of 31% to 92.9% in patients with PBC [25]. The difference in positive rate is associated with the detection method and sample size. Previous research demonstrated that the positive rate of ANA was higher in AMA-negative patients with PBC than in AMA-positive patients; however, some studies revealed that PBC-specific ANAs, such as nuclear pore membrane protein gp210 and nuclear body protein sp100, correlate with disease severity [26]. Notably, the rates of positive ANA were significantly higher in PBC patients with SS than in both PBC alone patients and SS alone patients. Meanwhile, it is agreed that hyperimmunoglobulinemia often occurs in SS patients, so the IgG levels were significantly higher in PBC+SS patients than in PBC-alone patients. Furthermore, anti-Ro/SSA antibodies are an important antibody in SS, with positive rates of approximately 75% [27]. This is an indication that clinicians should screen for CTDs, especially SS, in PBC patients with positive ANA or anti-Ro/SSA antibodies or elevated serum IgG levels, even in patients with early atypical symptoms of dry. Interestingly, serum AMA and AMA-M2 antibodies are specific autoantibodies for PBC, with positive rates of up to 94%, but higher positive rates of AMA and AMA-M2 were detected in the PBC+SS patients than in the PBC-alone patients [18]. A plausible possibility is that PBC accompanied by SS has an enhanced immune response, thus resulting in higher antibody positivity; however, the direct pathogenic role in PBC+SS patients with higher positive rates of AMA and AMA-M2 is still unclear and needs further study.
Our meta-analysis has some limitations. First, high heterogeneity is a general concern in epidemiological meta-analyses included in our meta-analysis. Second, observational studies predispose the aggregate results to be susceptible to selection bias. Third, the different diagnostic criteria, because there is no clear sign of SS were used among these included studies, thus influencing the number of patients with SS and representing certain publication bias in the results of the proportional meta-analysis. In addition, there are large differences in sample sizes, leading to a dilution effect on the meta-analysis. Finally, limited data were available on the treated response and clinical outcomes of PBC patients with concomitant SS; however, in fact, our main aim is to identify the potential impact of PBC on outcomes affected by concomitant SS. For these reasons, these findings should be interpreted prudently.
5ConclusionsIn conclusion, this review highlights that comorbid SS is very common in patients with PBC, affecting 35% of patients. In addition, there were some differences in the biochemical responses and immunological indicators of the PBC and PBC with SS groups. In particular, PBC+SS patients have lower ALB and PLT, and the presence of advanced fibrosis is much more likely in these patients. However, further studies with more strictly randomized controlled trials and larger sample sizes are needed to explain the clinical significance of these differences.
FundingThis work was supported by the Natural Science Foundation of Hubei Province (2020CFB774) and the National Natural Science Foundation of China (81800519).
Data availability statementData is openly available in a public repository.
Authors’ contributionsXiaoling Deng: conceptualization; Jiahuan Li, Shuhui Hou, Bai Ci, and Beibei Liu: data curation, formal analysis, software, visualization, and methodology; Xiaoling Deng, and Keshu Xu: project administration and resources; Xiaoling Deng, and Jiahuan Li: writing and editing; Jiahuan Li, and Keshu Xu: supervision. All the authors gave their final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Supplementary Fig. S1 Funnel plots of publication bias. A: Publication bias of pooled prevalence of SS in PBC patients; B: Publication bias of ALT; C: Publication bias of AST; D: Publication bias of ALP; E: Publication bias of γ-GT; F: Publication bias of TBiL; G: Publication bias of ALB; H: Publication bias of PLT; I: Publication bias of IgG; J: Publication bias of IgM; K: Publication bias of ANA; L: Publication bias of AMA; M: Publication bias of AMA-M2; N: Publication bias of anti-Ro/SSA antibodies. ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase, γ-GT, γ-glutamyltransferase; TBiL, total bilirubin; ALB, albumin; PLT, platelet; ANA, antinuclear antibody; AMA, anti-mitochondrial antibody; SSA, Sjögren's syndrome antigen A; SE, standard error; MD, mean difference; RR, relative risk.