We read the new nomenclature for steatotic liver disease (SLD) with great interest [1]. Given the existence of phenotypic heterogeneity of fatty liver, the extent to which these innovative SLD phenotypes (i.e., metabolic dysfunction-associated steatotic liver disease [MASLD], alcohol-related liver disease [ALD], and an overlap of the 2 [MetALD]) were associated with adverse events remains unclear.
We utilized data from the Third National Health and Nutrition Examination Survey (NHANES III), which comprised data on ultrasonography-measured steatosis [2]. The ethical review board of the National Center for Health Statistics approved the implementation of NHANES. Each participant provided information on alcohol consumption through a questionnaire [2]. In NHAENS, a drink means a 12-oz beer, a 4-oz glass of wine, or an ounce of liquor, which could be converted to 14 grams of pure alcohol [3]. In this study, as suggested by the consensus [1], MetALD patients were classified into a category distinct from MASLD to capture the pathogenic value of alcohol consumption and prognostic implications [1]. Participants were followed for the all-cause mortality by the National Death Index records were reviewed on December 31, 2019.
Our analysis included 6279 participants from NHANES III who were then categorized into five groups: (1) MASLD, N=872, (2) MetALD, N=141, (3) ALD, N=64, (4) Other SLD, N=108, and (5) Participants without hepatic steatosis, N=5094. We used a multivariable-adjusted Cox proportional hazard regression model to assess the association of these SLD phenotypes with all-cause mortality, with models accounting for demographic and cardiometabolic risk factors. P values were 2-sided and considered significant at 0.05. All analyses were performed by using R, version 4.2.1.
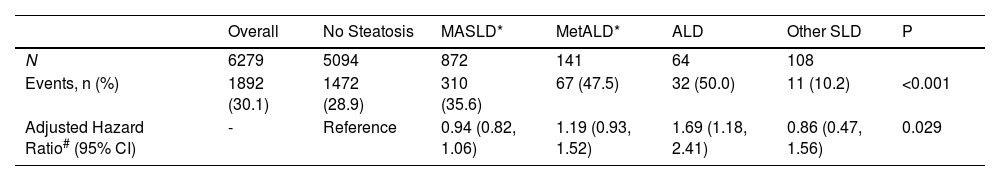
As shown in Table 1, in this large and nationally representative US cohort consisting of 6279 adults (age 48.1±20, 53 % female), the prevalence of MASLD was 13.8 %, MetALD was 2.2 % and ALD was 1.0. After a median follow-up of 26.9 years, 1892 all-cause deaths were documented. We observed that ALD was significantly associated with a 69 % higher hazard of all-cause mortality (hazard ratio [95 % confidence interval], 1.69 [1.18–2.41], P<0.001) when compared with healthy controls, while the association between other SLD phenotypes and mortality are neutral (P for all >0.1).
Prevalence and mortality prognosis of steatotic liver disease phenotypes.
| Overall | No Steatosis | MASLD* | MetALD* | ALD | Other SLD | P | |
|---|---|---|---|---|---|---|---|
| N | 6279 | 5094 | 872 | 141 | 64 | 108 | |
| Events, n (%) | 1892 (30.1) | 1472 (28.9) | 310 (35.6) | 67 (47.5) | 32 (50.0) | 11 (10.2) | <0.001 |
| Adjusted Hazard Ratio# (95% CI) | - | Reference | 0.94 (0.82, 1.06) | 1.19 (0.93, 1.52) | 1.69 (1.18, 2.41) | 0.86 (0.47, 1.56) | 0.029 |
MASLD, metabolic dysfunction-associated steatotic liver disease; ALD, alcohol-related liver disease; CI, confidence interval.
The five cardiometabolic criteria evaluated as specified by Rinella et al. (1) Systolic blood pressure ≥ 130 mmHg or diastolic ≥ 85 mmHg, or use of antihypertensive medication. (2) Blood glucose ≥ 100 mg/dL or HbA1c ≥ 6.5%, or presence of type 2 diabetes or diabetes treatment. (3) BMI ≥ 25 or waist circumference > 94 cm (males), > 80 cm (females). (4) HDL cholesterol ≤ 40 mg/dL (males), ≤ 50 mg/dL (females), or lipid-lowering treatment. (5) Triglycerides ≥ 150 mg/dL or intake of lipid therapy.
Other SLD represents those who had non-alcoholic SLD without metabolic dysfunction.
Previous studies have demonstrated the association between metabolic dysfunction associated with fatty liver disease and all-cause mortality [2]. However, as metabolic dysfunction related to fatty liver disease does not entirely exclude ALD [4], the role of alcohol intake in SLD patients remains unclear. Our study expanded prior findings and, for the first time, showed that, for SLD patients, alcohol intake may play a more critical role than metabolic dysfunction in association with all-cause mortality. Considering that alcohol consumption was one of the leading causes of death and disability worldwide [5]. Our study further highlights the impact of alcohol consumption on mortality in SLD patients. Restricting alcohol consumption may be a crucial measure in reducing the mortality of SLD patients. The new nomenclature may provide important insights into risk stratification from an alcohol-related pathophysiological pathway. It should be noted that the population studied in the NHANES III database was examined between 1988 and 1994; the dynamic evolution of SLD during follow up was not captured in this study. More studies are warranted to validate our findings.
Author contributionsHJ, ZS, JG-Conceptualization, HJ, ZS - Data curation, HJ, ZS- Formal analysis, HJ- Funding acquisition, All authors- Investigation, All authors - Methodology, JG - Project administration, JG, HJ- Resources, HJ, ZS- Software, HJ- Supervision, HJ- Validation, HJ- Visualization, All authors- Writing - original draft, All authors - Writing - review & editing.
FundingThis study was funded in part by the National Natural Science Foundation of China (Grant ID. 82103908), the Taishan Scholar Program of Shandong Province (tsqn202211364) and the Shandong Provincial Natural Science Foundation (ZR2021QH014). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declaration of interestsNone.