Acute on Chronic Liver Failure (ACLF) is characterized by organ failure and high 28-day mortality. Identifying clinical predictors associated with early mortality could have implications for the treatment of patients with ACLF.
Patients and methodsPatients diagnosed with chronic liver failure that developed ACLF based on the EASL-CLIF Consortium definition admitted to the Intensive care unit of a tertiary hospital between 2012–2018 were included. Bivariate and multivariate Cox regression analyses were performed to identify factors associated with mortality.
Results148 patients (55% female) were diagnosed with ACLF of which 55% (n = 82) had ACLF grade 3, 28% (n = 41) grade 2 and 17% (n = 25) grade 1. The median age was 54 years (41-63). Hepatitis C virus (HCV) was the most frequent etiology in 29.8% (n = 44) of the patients with bacterial infection being the most predominant precipitant factor in 58.1% (n = 86). Ninety-day global cumulative survival was only 18%. When divided by grade, mortality reached to 10% in ACLF 3. Moreover, in the multivariate Cox regression analysis, renal failure (HR 3.26, 95% CI (2.13–4.99), brain failure (HR 1.37, 95% CI 1.09–2.04) and male sex (HR 1.62, 95% CI 1.10–2.40) were independent predictors of 28- and 90-day mortality.
ConclusionsACLF is a frequent syndrome among chronic liver disease patients. Brain and renal failure are significantly associated with higher mortality and are independent predictors of 28 and 90-day mortality.
Acute on chronic liver failure (ACLF) is a clinical syndrome manifesting as an acute insult on an already compromised liver with a chronic disease that is associated with organ failure and high short-term mortality [1].
Multiple organ dysfunction is a common cause of morbidity and mortality in intensive care units (ICUs) [2] and in patients with ACLF [3]. It is known that bacterial infections which are the most common precipitant in ACLF patients, are associated with a bad prognosis [1,4], and with the development of organ failure(s) [5–7]. ACLF events are classified based on the number of organ failure (s) (OFs), grade 1 (less severe) to grade 3 (most severe) and it is universally known that short-term survival worsens with increasing ACLF grades [1,8].
Moreover, not only the number of organs involved but the type of organ affected, has an impact on mortality [9,10]. Hepatic encephalopathy (HE) grade 3–4 has been associated with high mortality in ACLF patients in the United States [9], while in Europe, renal failure in ACLF patients has a high short-term mortality [11]. Renal failure has also been demonstrated to be a negative predictor of ACLF resolution [12]. Whereas liver failure within the CLIF-C ACLF score, has been proven to independently predict a severe course [13].
While most of the information we currently have regarding ACLF comes from the European, American and Asian cohorts, information on ACLF and predictors of mortality on Latin American population are completely lacking [1,14,15]. Here we present our 6-year single-center experience of patients admitted to the ICU that fulfill the criteria for ACLF, and describe the clinical factors and type of organ failures that are independent predictors of 28 and 90-day mortality.
2Methods2.1PatientsPatients with cirrhosis that were admitted to the intensive care unit (ICU) due to a decompensation event between 2012 to 2018 and fulfilled criteria for ACLF diagnosis within the first 6 days of hospitalization were included. The diagnosis of liver cirrhosis was based on histological, clinical signs of hepatic decompensation (HE, variceal bleeding, jaundice, ascites and/or hepatorenal syndrome) ultrasonography, and/or endoscopic findings. Liver disease severity was assessed using the following scores: Child-pugh, Model for End-Stage liver disease (MELD) [16,17]. We excluded patients younger than 18 years old, pregnant, and patients diagnosed with hepatocellular carcinoma, as well as those who received a liver transplant during the 90-day follow-up after the diagnosis of ACLF. The present study was approved by the ethics committee and was performed according to the ethical guidelines of the 1975 Declaration of Helsinki. The requirement for obtaining informed consent from patients was waived because of the retrospective nature of the study.
2.2ACLF and organ failure definitionsACLF was diagnosed according to the EASL-CLIF Consortium criteria [1]. Grading of ACLF was performed according to the CANONIC study [1] with the following classification: ACLF 1: patients with renal failure (creatinine ≥2.0 mg/dl) or a non-renal organ failure plus renal dysfunction (creatinine between 1.5–1.9 mg/dl) and/or HE grade I–II. ACLF 2: Patients with 2 organ failures; ACLF 3: Patients with 3 or more organ failures. Organ Failure(s) were defined as follows: Liver (serum bilirubin ≥12.0 mg/dl), renal (creatinine ≥2 mg/dl or renal replacement), brain (HE III–IV grade), coagulation (INR ≥ 2.5), circulatory (vasopressor use for circulatory failure indication) and lung (SpO2/FiO2 ≤ 214 or mechanical Ventilation for lung failure).
2.3InfectionsThe following diagnostic criteria for infection were used: suggestive findings on chest radiographs was diagnostic of pneumonia [18], Spontaneous Bacterial Peritonitis (SBP) was based on an increased number of polymorphonuclear neutrophils in ascitic fluid (>250/mm3) [19] ;more than 15 white blood cells in urine per high power field with either positive urine gram stain or culture was diagnostic of urinary tract infection [20]; and acute cholangitis was suspected clinically by the Charcot triad (pain, fever, jaundice) and confirmed by means of biliary tract dilatation or obstruction [21]. Nosocomial infections were those diagnosed after 48 h of admission, and community acquired (CA) within 48 h of admission. Diagnosis of other infections were made according to conventional criteria [22].
2.4Data collectionThe following data were collected from the charts: patient demographics including age, sex, cirrhosis etiology, ascites according to the International Club of Ascites [23], HE according to the West-Haven criteria [24], vital signs, biochemical profile including complete blood count (CBC), liver function tests, use of vasopressors, need for renal replacement therapy, mechanical ventilation and precipitating factors (Infection, GI bleeding, HE, Alcohol) affecting ACLF development and mortality. Patients were followed for up to 90-days. If patients were discharged before 90-days, the follow-up was performed by reviewing the medical records, contacting the patient, a close relative or legal representative, or in person during a scheduled follow-up appointment.
2.5Statistical analysisAll results were expressed as mean ± standard deviation (SD) or median and interquartile range (percentile 25 to percentile 75) according to the normal distribution of the data. The quantitative variable distribution was performed using asymmetry values, kurtosis, and Kolmogorov–Smirnov test. Categorical variables were reported in frequencies and percentages and compared using Pearson’s Chi-squared test. The comparison among ACLF grades was performed using ANOVA when the continuous variables were normally distributed or a non-parametric test (Kruskal–Wallis) for those variables with asymmetric distribution. We constructed a Cox regression analysis to evaluate the possible predictors/associations with ACLF mortality. We used a stepwise approach and included only clinically meaningful variables in the final model to understand what factors were independently associated with 28 and 90-day mortality. Collinearity between explanatory variables was tested using the Variance Inflation Factor, highly correlated variables were excluded from the analysis to prevent issues with multicollinearity. Survival curves were done using a Kaplan–Meier plot and were compared with the log-rank test. All analyses were performed using SPSS (Statistical Package for the Social Sciences) version 22 and GraphPad Prism version 8 software. A p-value of <0.05 was considered statistically significant.
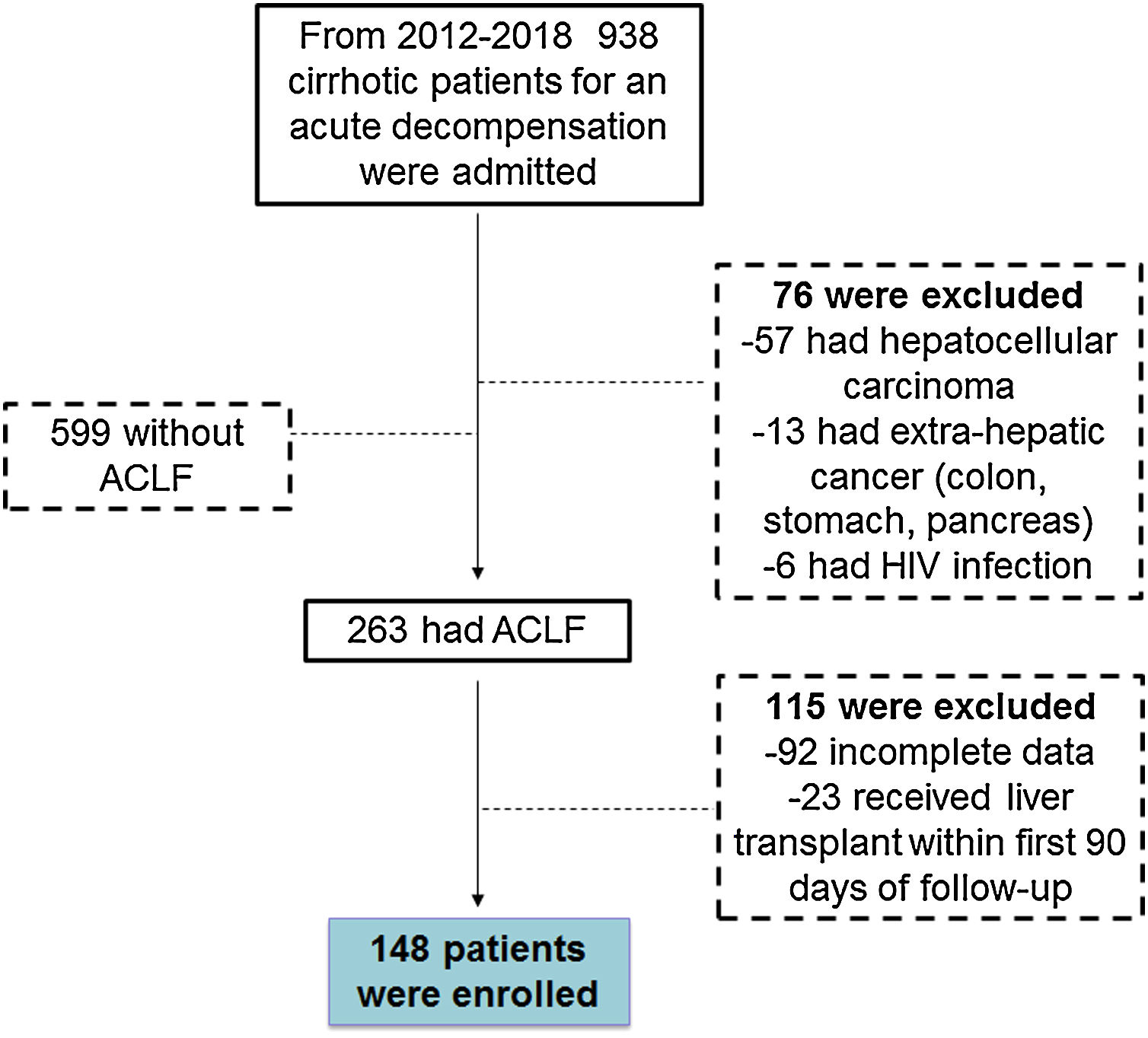
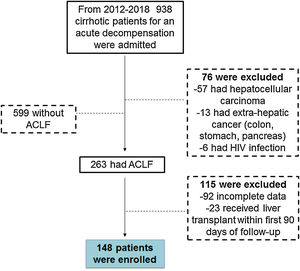
3Results3.1General demographicsFrom 2012–2018 a total of 938 cirrhotic patients were seen in the emergency department and admitted to the ICU due to a decompensation event. Of those 938 patients, 599 had decompensated cirrhosis (DC), 263 developed ACLF and 76 patients were excluded for the following reasons (57 had hepatocellular carcinoma, 13 had extra-hepatic cancer (colon, stomach, pancreas) and 6 had HIV infection). From the 263 patients fulfilling ACLF inclusion criteria, 115 were excluded (92 had incomplete data and 23 received liver transplantation during the 90 days following the diagnosis of ACLF). Thus, a total of 148 patients were included in this study, with the majority fulfilling ACLF criteria either at the time of admission or during the first 6 days of admission to the ICU (Fig. 1).
The median age for our cohort was 54 years old (interquartile range 41–63 years). Sex frequency was very similar, with females constituting 55% (n = 81) of the population. The underlying causes of cirrhosis were virus 29.8% (n = 44), cryptogenic 17.6% (n = 26), alcohol 13.5% (n = 20), Autoimmune Hepatitis (AIH) 11.5% (n = 17), Primary Biliary Cirrhosis (PBC) 8.1% (n = 12), and non-alcoholic fatty liver disease (NAFLD) 7.4% (n = 11). Based on the liver disease severity scores, the majority of the patients were severely ill, had MELD score >15 (69.6%; n = 103), 68.2% (n = 101) had Child-Pugh C, 28.4 % (n = 42) had Child-Pugh B and only 3.4% (n = 5) had Child-Pugh A. In addition, more than half of the patients 55% (n = 82) had ACLF grade 3, 28% (n = 41) grade 2, and only 17% (n = 25) grade 1 (Supplementary Table S1).
The most common precipitating events were infection 58.1% (n = 86), GI bleeding 8.1% (n = 12), Hepatic Encephalopathy (HE) 2.7% (n = 4) and alcohol 2.7% (n = 4). Interestingly in 28.4% (n = 42) of the patients, the precipitant was unknown (Supplementary Table S1).
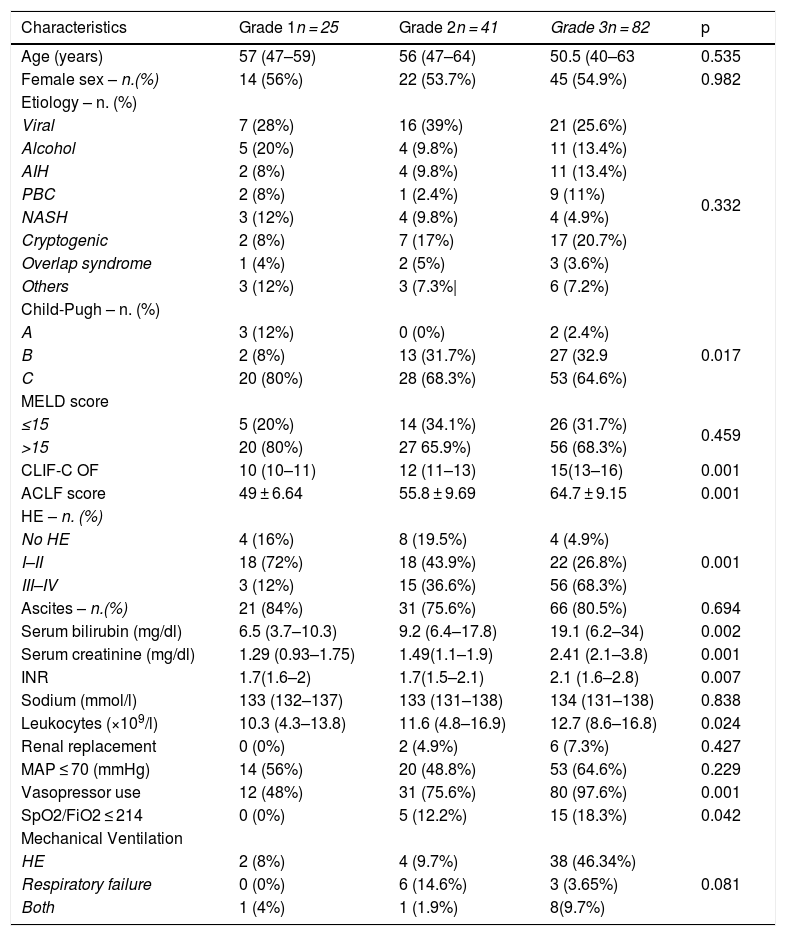
3.2Clinical and biochemical characteristics and organ failure prevalence based on ACLF gradingPatients were further classified into ACLF grade, and clinical and biochemical characteristics were analyzed (Table 2). There were no statistical differences in age, sex or etiology among the different ACLF grades (p = 0.535), (p = 0.982), (p = 0.332) respectively. However, we found a significant difference among them in the presence of HE (p = 0.001), bilirubin (p = 0.002), creatinine (p = 0.001), INR (p = 0.007), leukocytes (p = 0.024), Child-Pugh score (p = 0.017) vasopressor use (P = 0.001) and SpO2/FiO2 ≤ 214 (p = 0.042). The majority of patients with ACLF grade 3 accounted for these differences, with 68.3% (n = 56) presenting with HE grades III–IV, a median of serum bilirubin of 19.1 mg/dl, creatinine of 2.41 mg/dl, INR of 2.1 mg/dl and total leukocyte count of 12.7 × 109/l. Sixty four percent (n = 53) of patients with ACLF-3 presented with severe circulatory failure with a MAP ≤ 70 (mmHg), and almost all of these patients, 97.6% (n = 80) required vasopressors. Likewise, mechanical ventilation was required in more than half of ACLF-3 patients 59.69% (n = 49), of which in 46.34% (n = 38) was indicated by HE. As we expected, the ACLF and CLIF-C OF scores were significantly different among groups with a proportional increase according to the degree of ACLF severity (p = 0.001) (Table 1).
Population baseline characteristics based on ACLF grading (n = 148).
| Characteristics | Grade 1n = 25 | Grade 2n = 41 | Grade 3n = 82 | p |
|---|---|---|---|---|
| Age (years) | 57 (47–59) | 56 (47–64) | 50.5 (40–63 | 0.535 |
| Female sex – n.(%) | 14 (56%) | 22 (53.7%) | 45 (54.9%) | 0.982 |
| Etiology – n. (%) | ||||
| Viral | 7 (28%) | 16 (39%) | 21 (25.6%) | 0.332 |
| Alcohol | 5 (20%) | 4 (9.8%) | 11 (13.4%) | |
| AIH | 2 (8%) | 4 (9.8%) | 11 (13.4%) | |
| PBC | 2 (8%) | 1 (2.4%) | 9 (11%) | |
| NASH | 3 (12%) | 4 (9.8%) | 4 (4.9%) | |
| Cryptogenic | 2 (8%) | 7 (17%) | 17 (20.7%) | |
| Overlap syndrome | 1 (4%) | 2 (5%) | 3 (3.6%) | |
| Others | 3 (12%) | 3 (7.3%| | 6 (7.2%) | |
| Child-Pugh – n. (%) | ||||
| A | 3 (12%) | 0 (0%) | 2 (2.4%) | 0.017 |
| B | 2 (8%) | 13 (31.7%) | 27 (32.9 | |
| C | 20 (80%) | 28 (68.3%) | 53 (64.6%) | |
| MELD score | ||||
| ≤15 | 5 (20%) | 14 (34.1%) | 26 (31.7%) | 0.459 |
| >15 | 20 (80%) | 27 65.9%) | 56 (68.3%) | |
| CLIF-C OF | 10 (10–11) | 12 (11–13) | 15(13–16) | 0.001 |
| ACLF score | 49 ± 6.64 | 55.8 ± 9.69 | 64.7 ± 9.15 | 0.001 |
| HE – n. (%) | ||||
| No HE | 4 (16%) | 8 (19.5%) | 4 (4.9%) | 0.001 |
| I–II | 18 (72%) | 18 (43.9%) | 22 (26.8%) | |
| III–IV | 3 (12%) | 15 (36.6%) | 56 (68.3%) | |
| Ascites – n.(%) | 21 (84%) | 31 (75.6%) | 66 (80.5%) | 0.694 |
| Serum bilirubin (mg/dl) | 6.5 (3.7–10.3) | 9.2 (6.4–17.8) | 19.1 (6.2–34) | 0.002 |
| Serum creatinine (mg/dl) | 1.29 (0.93–1.75) | 1.49(1.1–1.9) | 2.41 (2.1–3.8) | 0.001 |
| INR | 1.7(1.6–2) | 1.7(1.5–2.1) | 2.1 (1.6–2.8) | 0.007 |
| Sodium (mmol/l) | 133 (132–137) | 133 (131–138) | 134 (131–138) | 0.838 |
| Leukocytes (×109/l) | 10.3 (4.3–13.8) | 11.6 (4.8–16.9) | 12.7 (8.6–16.8) | 0.024 |
| Renal replacement | 0 (0%) | 2 (4.9%) | 6 (7.3%) | 0.427 |
| MAP ≤ 70 (mmHg) | 14 (56%) | 20 (48.8%) | 53 (64.6%) | 0.229 |
| Vasopressor use | 12 (48%) | 31 (75.6%) | 80 (97.6%) | 0.001 |
| SpO2/FiO2 ≤ 214 | 0 (0%) | 5 (12.2%) | 15 (18.3%) | 0.042 |
| Mechanical Ventilation | ||||
| HE | 2 (8%) | 4 (9.7%) | 38 (46.34%) | 0.081 |
| Respiratory failure | 0 (0%) | 6 (14.6%) | 3 (3.65%) | |
| Both | 1 (4%) | 1 (1.9%) | 8(9.7%) | |
The data are presented in frequency (n.) and percentage (%), median and interquartile range, mean and standard deviation (SD)(±). Abbreviations. ACLF (Acute on chronic liver failure), AIH (Autoimmune Hepatitis), PBC (Primary Biliary Cholangitis), NAFLD (non-alcoholic fatty liver disease), MELD (Model for End-stage Liver Disease), CLIF-C OF(Chronic Liver Failure -Organ Failure), Mg (milligram), HE (Hepatic encephalopathy), dl (deciliter) mmol/l (milliosmoles/liter), MAP (Mean arterial pressure), SpO2 (Oxygen saturation), FiO2 (Fractional inspired oxygen).
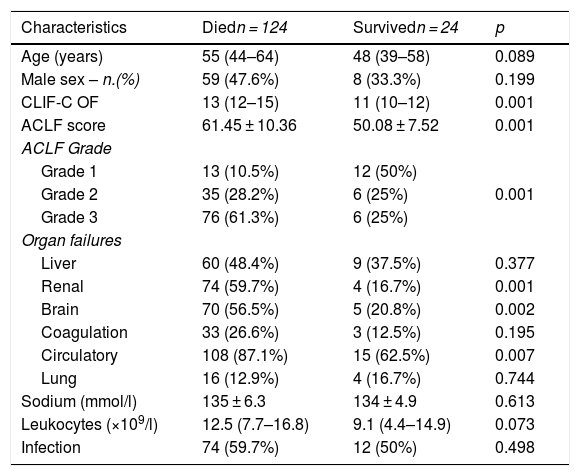
General characteristics based on outcome.
| Characteristics | Diedn = 124 | Survivedn = 24 | p |
|---|---|---|---|
| Age (years) | 55 (44–64) | 48 (39–58) | 0.089 |
| Male sex – n.(%) | 59 (47.6%) | 8 (33.3%) | 0.199 |
| CLIF-C OF | 13 (12–15) | 11 (10–12) | 0.001 |
| ACLF score | 61.45 ± 10.36 | 50.08 ± 7.52 | 0.001 |
| ACLF Grade | |||
| Grade 1 | 13 (10.5%) | 12 (50%) | 0.001 |
| Grade 2 | 35 (28.2%) | 6 (25%) | |
| Grade 3 | 76 (61.3%) | 6 (25%) | |
| Organ failures | |||
| Liver | 60 (48.4%) | 9 (37.5%) | 0.377 |
| Renal | 74 (59.7%) | 4 (16.7%) | 0.001 |
| Brain | 70 (56.5%) | 5 (20.8%) | 0.002 |
| Coagulation | 33 (26.6%) | 3 (12.5%) | 0.195 |
| Circulatory | 108 (87.1%) | 15 (62.5%) | 0.007 |
| Lung | 16 (12.9%) | 4 (16.7%) | 0.744 |
| Sodium (mmol/l) | 135 ± 6.3 | 134 ± 4.9 | 0.613 |
| Leukocytes (×109/l) | 12.5 (7.7–16.8) | 9.1 (4.4–14.9) | 0.073 |
| Infection | 74 (59.7%) | 12 (50%) | 0.498 |
The data are presented in frequency (n.) and percentage (%), median and interquartile range (P25–P75), mean and standard deviation (SD) (±). Bivariate analysis by means of U Mann–Whitney test, T-student and chi square test. Abbreviations. CLIF-C OF (Chronic Liver Failure - Organ Failure) ACLF (Acute on chronic liver failure).
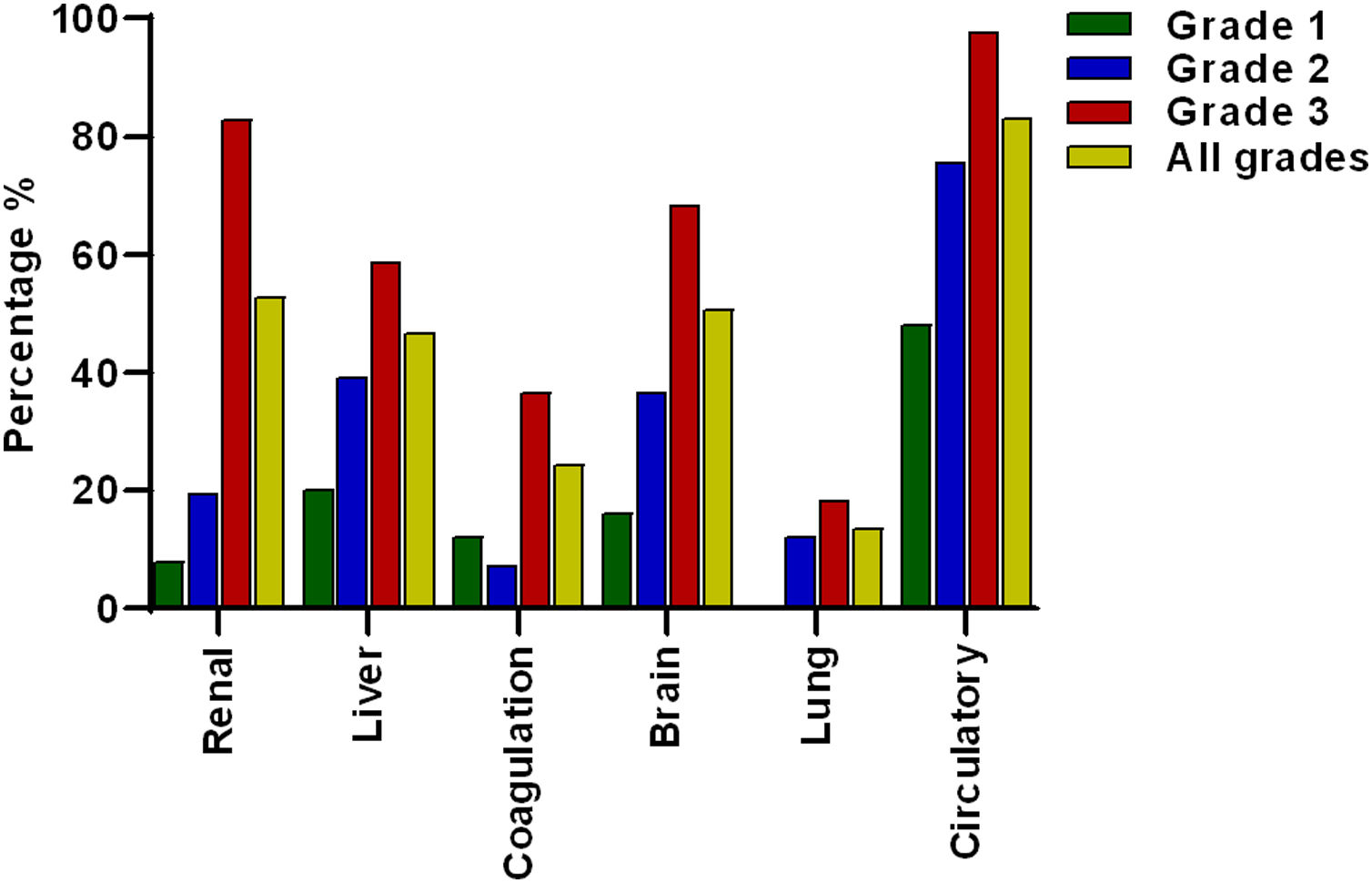
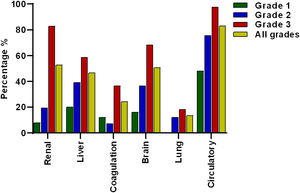
The most prevalent OF was the circulation with 83.1% (n = 123) of the total cases, followed by renal 52.7% (n = 78), brain 50.7% (n = 75), liver 46.6% (n = 69), coagulation 24.3% (n = 36) and lung failure 13.5% (n = 20), of cases respectively (Fig. 2).
Frequency of organ failures based on ACLF grading. Circulatory, renal and brain failure were the most prevalent organ failures. According to the ACLF grade, circulatory failure was the most prevalent in all ACLF grades. Lung failure was not present in grade 1 and renal failure was more prevalent in grade 3 compared to grade 2 and 1.
When patients were divided according to ACLF severity, it was interesting to note that in ACLF 1 there was no lung failure. Meanwhile, the failures that occurred most frequently in grade 3 ACLF were the circulatory 97.6% (n = 80) and renal failure 82.9% (n = 68) (Fig. 2).
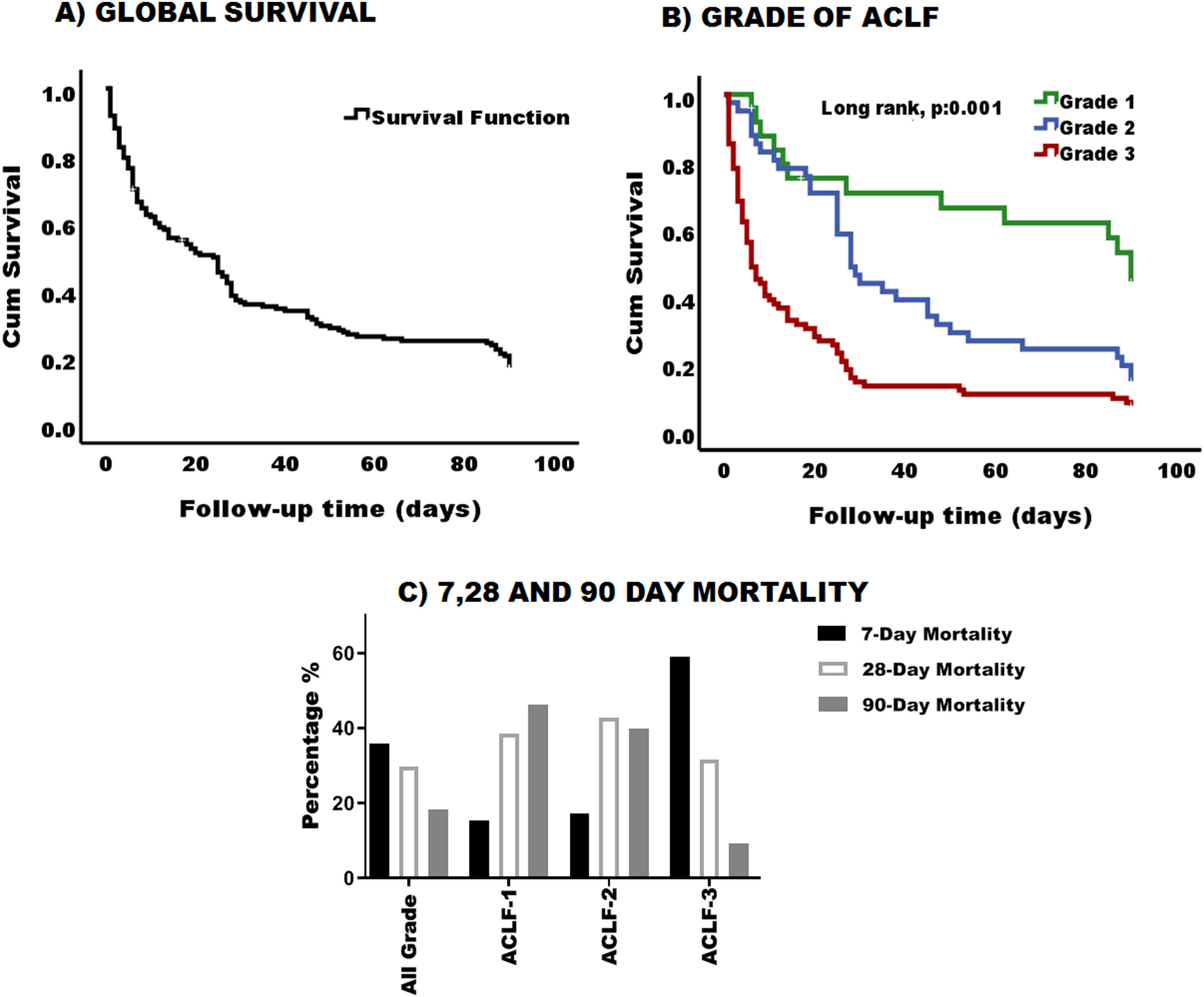
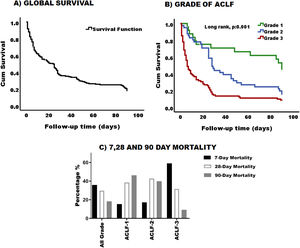
3.3Survival and mortality according to ACLF gradingThe global 90-day survival for patients with ACLF was 18% (Fig. 3-A) and the median overall survival was 19 days 95% CI (11.96–26.03). When survival was analyzed according to ACLF severity, we found that the higher the ACLF grade, the lower the survival (Fig. 3-B). Survival for patients with ACLF-3 was extremely low 10%, while survival for grades 2 and 1, was 22% and 45% respectively. Likewise, the median survival in grade 3 was 6 days, 95% CI (3.3–8.6), grade 2 was 28 days, 95% CI (22.7–33.29) and grade 1 was 90 days 95% CI (82.3–97.65). There was a statistical difference in the survival curves between ACLF grades (p = 0.001) (Fig. 3-B).
Survival and mortality in patiens with ACLF. (A) Graphic representation of cumulative survival during the 90-day follow up. After 90 days, only 18% of the cohort was still alive. (B) Survival was greater in patients with grade 1 and decreased as the degree of ACLF increases (log rank, p:0.001). Green line: Grade 1, blue line: Grade 2, and red line: Grade 3. (C) Percentage of 7, 28- and 90-day mortality based on ACLF grading.
When mortality was analyzed globally, it was clear that most of the patients died within the first 7 days 35.8% (n = 53); 29.7% (n = 44) of patients died by 28 days and 18.2% (n = 27) by 90 days.
According to the ACLF grade, as expected, ACLF grade 3 patients had higher mortality within the first 7 days [59.2% (n = 45)], compared to grade 2 [17.1% (n = 6)] and grade 1 [15.4% (n = 2)]. The same applied for 28 and 90 days with 31.6% (n = 24) and 9.2% (n = 7) of cases respectively (Fig. 3-C).
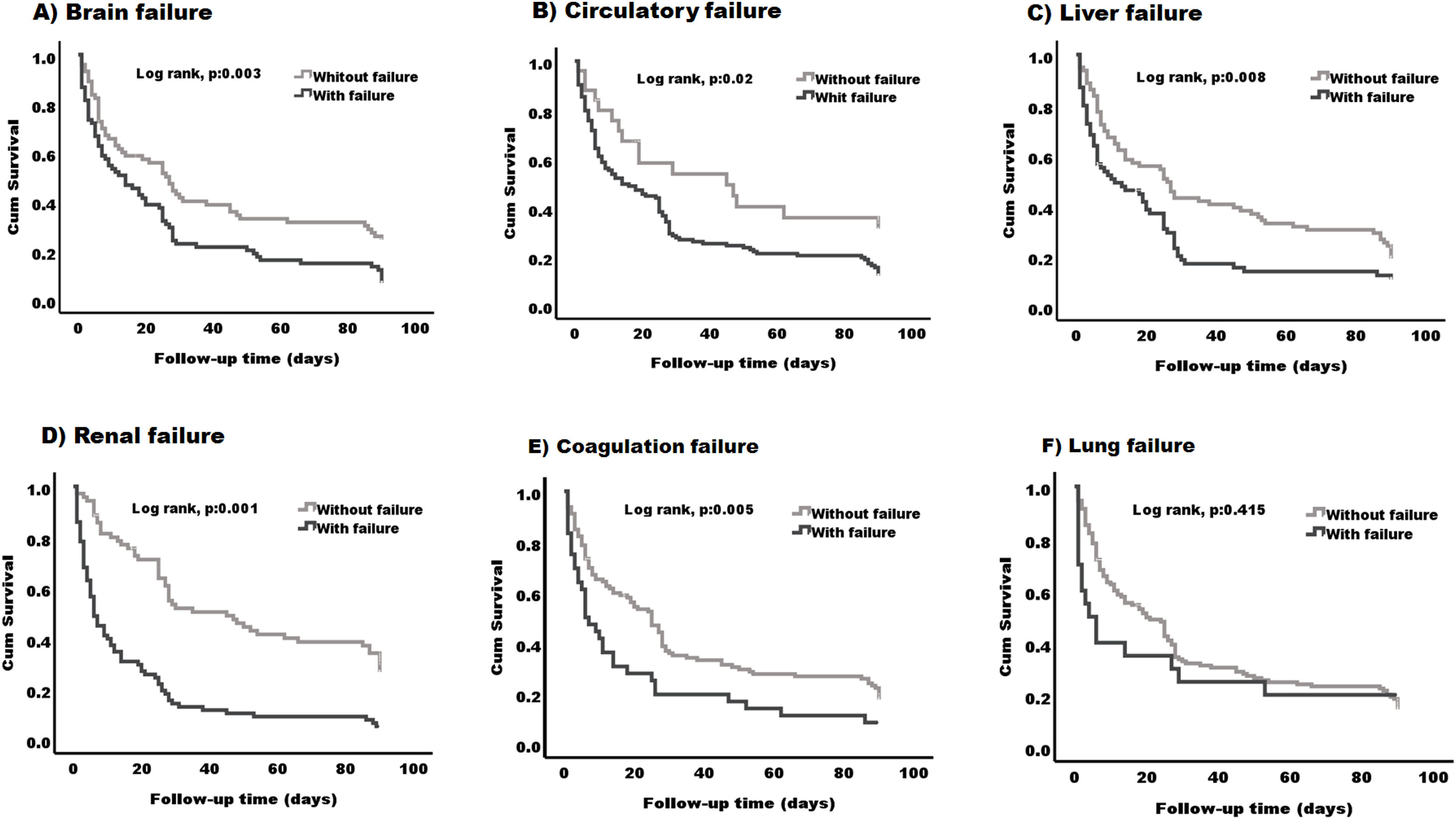
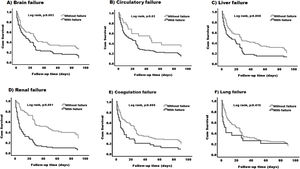
We next evaluated which organ failure was associated with lower survival. When comparing patients with and without each of the different organ failures, it was clear that having brain, circulatory, liver, renal or coagulation failure was associated with lower survival (P < 0.05) (Fig. 4-A–E). The median survival was lower in patients with the presence of any of the specified organ failure than in those without (12 days, 95% CI (4.50–19.49) vs 27 days, 95% CI (19.74–34.25) brain), (14 days, 95% CI (4.80–23.19) vs 45 days, 95% CI (2.61–87.38) circulatory) (11 days, 95% CI (0.9–23.44) vs 26 days, 95% CI (17.29–34.71) liver), (6 days, 95% CI (4.07–7.92) vs 45 days, 95% CI (28.23–61.77) renal) (6 days 95% CI (2.08–9.92) vs 25 days, 95% CI (18.76–31.23) coagulation) (Fig. 4A–E) respectively. Lung failure was associated with the lowest median survival, however, there was no significant difference between patients with and without the failure (2.23 days, 95% CI (1–8.38) vs 20 days, 95% CI (12.52–27.47) (Fig. 4-F).
Kaplan–Meier survival curve in patients based on the type of organ failure. Patients with the following organ failures (A) brain failure (B) circulatory failure (C) liver failure (D) renal failure and (E) coagulation failure, have lower survival (log rank, p:0.001) - (log rank, p:0.02) compared to those without any of these organs. (F) No differences were found between patients with lung failure and those without (log rank, p:0.415).
We first divided patients between those who died (n = 124) and those who survived (n = 24) (Table 3). No significant differences were found in age, however the majority of patients who died were female (52.4% vs 47.6%). As expected, patients that died, had a significantly higher CLIF-C OF [13 (12–15) vs 11 (10–12) p = 0.001], and ACLF scores (61.45 ± 10.36 vs 50.08 ± 7.52) p = 0.001, when compared to those who survived. The majority of the patients that died had ACLF grade 3 [76 (61.3%) vs 6 (25%), p = 0.001] and a significantly greater proportion of circulatory failure [108 (87.1%) vs 15 (62.5%), p = 0.007], renal failure [74 (59.7%) vs 4 (16.7%), p = 0.001] and brain failure [70 (56.5%) vs 5 (20.8%), p = 0.002]. No significant differences were observed in coagulation failure [33 (26.6%) vs 3 (12.5%), p = 0.195], liver failure [60 (48.4%) vs 9 (37.5%), p = 0.377] and lung failure [16 (12.9%) vs 4 (16.7%), p = 0.744] between patients that died and those who survived. While no difference in infection was observed between dead and surviving patients [(74 (59.7%) vs 12 (50%), p = 0.498], leukocyte count was significantly higher in patients that died compared to those who survived [12.5 × 109/L (7.7–16.8) vs 9.1 × 109/L (4.4–14.9), p = 0.073] (Table 2).
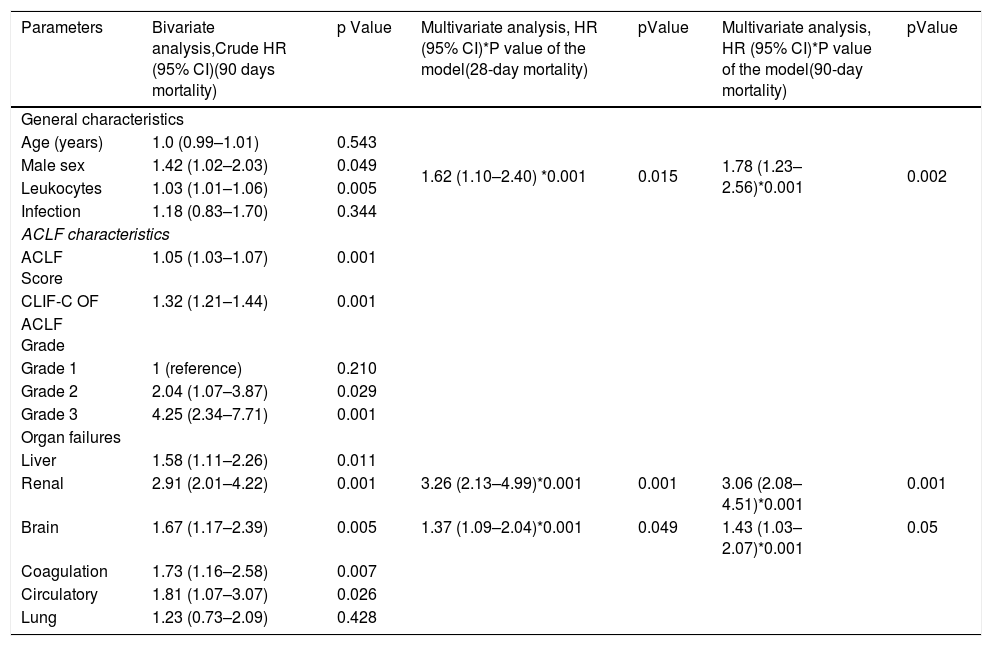
Clinical predictors of 28 and 90 day mortality. Bivariate and multivariate Cox regression analysis.
| Parameters | Bivariate analysis,Crude HR (95% CI)(90 days mortality) | p Value | Multivariate analysis, HR (95% CI)*P value of the model(28-day mortality) | pValue | Multivariate analysis, HR (95% CI)*P value of the model(90-day mortality) | pValue |
|---|---|---|---|---|---|---|
| General characteristics | ||||||
| Age (years) | 1.0 (0.99–1.01) | 0.543 | 1.62 (1.10–2.40) *0.001 | 0.015 | 1.78 (1.23–2.56)*0.001 | 0.002 |
| Male sex | 1.42 (1.02–2.03) | 0.049 | ||||
| Leukocytes | 1.03 (1.01–1.06) | 0.005 | ||||
| Infection | 1.18 (0.83–1.70) | 0.344 | ||||
| ACLF characteristics | ||||||
| ACLF Score | 1.05 (1.03–1.07) | 0.001 | ||||
| CLIF-C OF | 1.32 (1.21–1.44) | 0.001 | ||||
| ACLF Grade | ||||||
| Grade 1 | 1 (reference) | 0.210 | ||||
| Grade 2 | 2.04 (1.07–3.87) | 0.029 | ||||
| Grade 3 | 4.25 (2.34–7.71) | 0.001 | ||||
| Organ failures | ||||||
| Liver | 1.58 (1.11–2.26) | 0.011 | ||||
| Renal | 2.91 (2.01–4.22) | 0.001 | 3.26 (2.13–4.99)*0.001 | 0.001 | 3.06 (2.08–4.51)*0.001 | 0.001 |
| Brain | 1.67 (1.17–2.39) | 0.005 | 1.37 (1.09–2.04)*0.001 | 0.049 | 1.43 (1.03–2.07)*0.001 | 0.05 |
| Coagulation | 1.73 (1.16–2.58) | 0.007 | ||||
| Circulatory | 1.81 (1.07–3.07) | 0.026 | ||||
| Lung | 1.23 (0.73–2.09) | 0.428 | ||||
Bivariate and multivariate analysis using Cox proportional risks. Abbreviations. HR (Hazard ratio), CI (Confidence interval), ACLF (Acute on chronic liver failure), CLIF-OF (Chronic Liver Failure - Organ Failure).
Based on these data, we next performed a bivariate analysis to determine the clinical parameters associated with 90 day-mortality (Table 3). We found that male sex HR 1.42 (1.02–2.03) p = 0.049 and leukocyte count HR 1.03 (1.01–1.06) p = 0.005, were associated with high 90-day mortality. Likewise, ACLF HR 1.05 (1.03–1.07) p = 0.001 and CLIF-C OF HR 1.32 (1.21–1.44) p = 0.001 scores, ACLF 3 HR 4.25 (2.34–7.71) p = 0.001, ACLF 2 HR 2.04 (1.07–3.87) P = 0.029 as well as the presence of all organ failures: liver HR 1.58 (1.11–2.26) p = 0.011, renal HR 2.91 (2.01–4.22) p = 0.001, brain HR 1.67 (1.17–2.39) p = 0.005, coagulation HR 1.73 (1.16–2.58) p = 0.007 and circulatory HR 1.81 (1.07–3.07) p = 0.026 were all associated with high 90-day mortality. We next performed a multivariate regression analysis using Cox proportional hazard ratio, to determine which factors were independently associated with 28 and 90-day mortality. Results are shown in Table 4. Based on this model, we determined that male sex, brain and renal organ failures were independent predictors of 28 and 90-day mortality in patients with ACLF (Table 3). Of the three factors mentioned above, renal failure (HR 3.26 95% CI (2.13–4.99), p < 0.001, proved superior compared to male sex (HR 1.62 95% CI (1.10–2.40), p = 0.015 and brain failure (HR 1.37 95% CI (1.09–2.04), p = 0.049) in predicting 28 day mortality. Meanwhile, the same variables were also independent predictors of 90-day mortality; with renal failure (HR 3.06 95% CI (2.08–4.51), p < 0.001 still being superior compared to male sex (HR 1.78 95% CI (1.23–2.56), p < 0.002) and brain failure (HR 1.43 95% CI (1.03–2.07), p = 0.05 in predicting 90-day mortality (Table 3).
4DiscussionTo our knowledge, this is the first study to report an independent cohort with detailed analysis of ACLF syndrome in the Mexican population.
In Latin America, only 2 studies have described ACLF patients. The first one was a prospective Brazilian cohort of 192 patients with decompensated cirrhosis, 24% of those developing ACLF [25]. The second one was performed in Argentina [26], where 29% of patients admitted for AD were diagnosed with ACLF. When compared with the CANONIC study, despite race differences, it seems that the overall prevalence in Latin America remains pretty much within the range of 20–30%.
However, there seems to be a difference in the mortality rate, which vary significantly between races. For instance, mortality rate in Europe according to the CANONIC, is 33% and 51% at 28-days and 90 days [1], while in Asia is 41% and 63% [27] respectively. On the other hand, in the American continent we have data from the USA indicating that ACLF mortality ranges from 26% to 40% at day 28 and day 90 [28].
Based on the gathered information, it is clear that our ACLF population differs significantly from Europeans, Asians and Americans. ACLF in the Mexican population is a well-defined entity characterized by an extremely high 28-day (70.3%) and 90-day (81.8%) mortality which doubles the one generally observed in other cohorts. In addition, our population comprises patients usually presenting with a more severe disease state as determined by their higher MELD and ACLF-scores prompting their ICU admission.
When comparing the etiology of the underlying chronic disease with those reported in the CANONIC study, we observed that we have the same 2 most prevalent causes (HCV and alcohol), with the main difference being that in the European population, alcohol (60.3%) was almost 6 times higher than HCV (13%). In our population, HCV (29.8%) doubled the alcohol proportion (13.5%), possibly because HCV in Mexico is the most common cause of cirrhosis [29]. Another likely contributing factor is that the hospital where our study was conducted is a reference center for treating HCV infection.
However, while prevalence according to ACLF degree in studies conducted in Latin America [25,26] matches that of the CANONIC [1], with ACLF grade 1 being the most prevalent group, we found very different results. Most of our patients presented with more advanced cirrhosis stage (Child-Pugh C 68.2%) and had more organ failure(s) with a great majority presenting with ACLF grade 3.
The precipitating factors described in the literature vary widely depending on the geographical area. In the region of Asia and the Pacific, on which the guidelines of Asian Pacific Association for the Study of the Liver (APASL) are based, the main ACLF precipitant is HBV. In European countries, these viral etiologies are replaced mostly by non-viral insults, such as bacterial infections [15] similarly to what was reported in Latin America (Brazil and Argentina) and what we found in our population. Despite that, our population’s mortality is still extremely high.
We found a significant association between high 90-day mortality and several clinical factors including leukocyte count, as well as ACLF and CLIF-C OF scores and ACLF grade 3. Those factors were increased in patients that died, compared to those that survived, indicating the critically ill population that this cohort comprises. In addition, having any of the 6 organ failures recognized by the CANONIC for the definition of ACLF was also associated with a higher 90-day mortality. Of all those, lung failure was the only organ not associated with 90-day mortality (p = 0.428, HR 1.23, 95% CI: 0.73–2.09. Whereas renal, brain, coagulation and circulatory failure were all associated with a HR between 2.91 and 1.58 of 90-day mortality.
Indeed, the most affected organ in our cohort was the circulatory system (83.1%) even among the different grades of ACLF (48% in ACLF 1, 75.6% in ACLF 2, and 97.6% in ACLF 3). In comparison, in the CANONIC study, the kidney was the most affected organ (55.8%) followed by the liver (43.6%); while in North America the brain (55.7%) was the most frequent one.
The multivariate analysis leads us to identify that 3 specific factors were independently associated with 28 and 90-day mortality. The first one is male sex (p = 0.015, HR 1.62, 95% CI: 1.10–2.40) and (p = 0.002, HR 1.78, 95% CI: 1.23–2.56) for 28 and 90 days respectively, which we attribute to the fact that females seek earlier medical attention and are more compliant to medical treatment. Additionally, all those with alcoholic etiology (87%, n = 20) are men of whom 94.4% (n = 17) died. Although the sample size is too small to take into account the type of etiology as a prognostic factor, a trend in this variable was also found.
The other two factors that independently predicted 28 and 90-day mortality were renal and brain failure (p = 0.001, HR 3.26, 95% CI: 2.13–4.99) and (p = 0.049, HR 1.37, 95% CI: 1.09–2.04) for 28 and (p = 0.001, HR 3.06, 95% CI: 2.08–4.51) and (p = 0.05, HR 1.43, 95% CI: 1.03–2.07) for 90 day respectively.
Recently, Sawhney et al., demonstrated that ACLF survival, diminishes in relation to the presence of HE (ACLF with HE 35/53, [66%]; ACLF without HE, 16/48 [33%]; p = 0,002) and the grade of HE, grade 0–1, 16/48 [33%]; grade 2, 19/32 [59%]; grade 3–4, 16/21 [76%]; p = 0.002) [6]. Similar results were shown by Cordoba et al., and an additional association with increased systemic inflammatory response in ACLF patients with HE was found, as assessed by elevated C reactive protein levels (ACLF without HE, 27 (12–49 mg/dl); ACLF with HE, 32 (16–60 mg/dl); p < 0.0001) [30]. These findings support the ones in our study, where patient prognosis is negatively affected by the presence of brain failure as determined by the presence of grade III/IV HE. In addition, our results also indicate that patients that died have a more severe systemic inflammatory response compared to those who survived as indicated by higher leukocyte count p = 0.073, 12.5 × 109/l (7.7–16.8) vs 9.1 × 109/l (4.4–14.9) despite no significant differences in the presence of infection p = 0.498, 74 (59.7%) vs 12 (50%). Renal failure as demonstrated in the CANONIC has also been associated with a worse mortality and with a severe systemic inflammatory response [1].
The relation between the three independent predictors of mortality (male sex, brain and renal failure) with the systemic inflammatory response observed in patients that died, deserve further attention since it could have implications in the physiopathology of ACLF.
Limitations to our study include its retrospective nature and the fact that we only describe patients treated in a single third-level center, where there is a larger number of patients with this condition. Therefore, it is not possible to generalize the prevalence nationwide.
This study provides a comprehensive analysis of the clinical characteristics of the ACLF syndrome in Mexico, which due to its high mortality rate is considered an important area of research. We have identified three important independent prognostic factors associated with higher 28 and 90-day mortality, which could allow us to prioritize ACLF patients for general supportive care, disease-specific therapy, management of complications and, if needed, a timely referral for liver transplantation. Additional studies are needed to understand its pathophysiology and to develop novel diagnostic markers and therapeutic options for ACLF patients.
In conclusion, ACLF patients admitted to the ICU have a very high short-term mortality, especially when patients are male and present with brain and renal failure.AbbreviationsACLFAcute on chronic liver failureHEHepatic encephalopathyICUIntensive care unitCLIF-C OFChronic Liver Failure - Organ FailureOFOrgan failureCBCComplete blood countSDStandard deviationSPSSStatistical Package for the Social SciencesAIHAutoimmune HepatitisPBCPrimary Biliary CholangitisNAFLDNon-alcoholic fatty liver diseaseMELDModel for End-stage Liver DiseaseMAPmean arterial pressureEASL-CLIFEuropean Association for the Study of the Liver – Chronic Liver FailureHCVHepatitis C virusAPASLAsian Pacific Association for the Study of the LiverICUIntensive care unit
AbbreviationsACLF Acute on chronic liver failure Hepatic encephalopathy Intensive care unit Chronic Liver Failure - Organ Failure Organ failure Complete blood count Standard deviation Statistical Package for the Social Sciences Autoimmune Hepatitis Primary Biliary Cholangitis Non-alcoholic fatty liver disease Model for End-stage Liver Disease mean arterial pressure European Association for the Study of the Liver – Chronic Liver Failure Hepatitis C virus Asian Pacific Association for the Study of the Liver Intensive care unit
The authors have no conflicts of interest to declare.
Author contributionsOMG: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis.
DACR, EC, EAA, JPE: acquisition of data and drafting of the manuscript, administrative, technical and material support
NNA, AT: Study concept and design, drafting of the manuscript, critical revision of the manuscript and study supervision
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors thanks National Autonomous University of Mexico. Master’s and Doctoral Program in Medical, Dental and Health Sciences and National Council of Science and Technology (CONACyT) for the support provided.