Hepatocellular carcinoma is the fifth most common malignant neoplasm worldwide. Most patients are not candidates to surgical treatment. The prognosis of this neoplasm is poor, with an overall survival rate of 8 weeks in unresectable tumors. Estrogen receptors have been found in up to 33% of this tumors, reason why treatment with tamoxifen or progesterone compounds have been tried to diminish this neoplasm’s progression but its use remains controversial. In our institution, thirteen patients were treated with tamoxifen (2040 mg/day) and 26 received supportive measures only. The clinical and tumoral characteristics were similar in both groups. Survival in the Tamoxifen group was of 5.5 ± 1.7 months while in the supportive measures group was of 2.1 ± 0.5 months (p = 0.018). Other factors related to an increased survival were: female gender and the Okuda score; age, TNM and aFP were not related to survival. The multivariate analysis showed that treatment with tamoxifen duplicates survival independently of the tumoral stage and functional hepatic reserve. It seems that the benefit of treatment with tamoxifen is limited and is not associated to the presence of estrogen receptors. In our study a 69 year-old man with diagnosis of non-resectable hepatocellular carcinoma and negative estrogen receptors, was treated with tamoxifen with a partial response and an overall survival of 4 years until November 2005. Despite some case reports that have shown tumoral regression, while other studies do not report any survival benefits. It is important to identify patients that would benefit from treatment with tamoxifen.
Hepatocellular carcinoma (HCC) causes near one million deaths annually and is the fifth most prevalent neoplasm worldwide.1,2 Between 50 and 80% of the HCC cases worldwide are associated to liver cirrhosis.3
Nowadays, the only potentially curative treatment options for HCC are surgical. Nevertheless, few patients are adequate candidates to this type of procedure due to advanced liver disease, metastases and vascular involvement. In cases where unresectable disease is present the prognosis is poor with a medium survival of 8 weeks; these patients are candidates to palliative treatment only.4
The current inefficacy of the therapeutic modalities encourages the development of new strategies for the management of HCC, multiple regional therapies have been tried and systemic chemotherapy in several combinations, including 5-fluorouracil, doxorubicin, epirubicin, etoposide, cisplatin, mitoxantrone, interferon, capecitabine and some other treatments like thalidomide, octreotide, medroxiprogesterone or tamoxifen (TMX) with contradictory or unfavorable results.2,5
Estrogen receptors are identified in 33% approximately of HCC. Several hepatic carcinogenesis animal models and epidemiologic studies in humans have suggested a close relationship between sexual hormones and HCC. Estrogens could be both inductors and promoters of hepatic carcinogenesis. It is known that estrogens affect the morphology and function of the liver and they can cause hepatic adenomas in humans. These findings have led to an attempt to use TMX as a treatment for HCC. There are multiple reports of HCC regression secondary to the administration of TMX.6,7 Despite these findings its use remains controversial, some studies have reported a survival improvement,7-10 while others do not show any benefits of its use.10-14
Materials and methodsThe aim of this study was to retrospectively analyze patients from the National Institute of Medical Sciences and Nutrition Salvador Zubiran (INCMNSZ) with nonoperable HCC. We analyzed 39 medical records of patients with the diagnosis of HCC that were not candidates neither for surgical nor for ablative treatment in a ten year-period (19912000). Thirteen patients received treatment with TMX (20-40 mg/day) alone; and 26 patients, supportive-measures only. HCC was diagnosed either by histopathological analysis or by CT scan of the liver in association to alpha-fetoprotein (aFP) levels > 400 ng/mL. An analysis of potential prognostic factors such as age, gender, aFP levels, cirrhosis, albumin levels, bilirubin levels, Child score, TNM and Okuda staging system was performed.
ResultsThe general characteristics of the patients receiving TMX and with supportive-measures only are shown in Table I.Table II shows the comparison of the clinical and tumoral characteristics of the patients according to their group.
General characteristics of patients. HBV, hepatitis B virus; HCV, hepatitis C virus.
| Support measures (26) | Tamoxifen (13) | P | Total (39) | |
|---|---|---|---|---|
| Age (years) | 58.15 ± 2.9 | 57.77 ± 3.5 | 0.937 | 58 ± 2.2 |
| Gender (M/F) | 14/12 | 7/6 | 1 | 21/18 |
| • Male (%) | 54 | 54 | 54 | |
| Cirrhosis (%) | 46 | 69 | 0.173 | 53 |
| Cirrhosis etiology% | ||||
| • Alcoholic | 2 5 | 0 | 0.263 | 1 4 |
| • HBV | 8 | 22 | 10 | |
| • HCV | 42 | 67 | 57 | |
| • Cryptogenic | 25 | 11 | 19 | |
| Classification Child-Pugh (%) | ||||
| • A | 23 | 39 | 0.466 | 28 |
| • B | 46 | 46 | 46 | |
| • C | 31 | 15 | 26 | |
| Serum Bilirubin (mg/dL) | 3.1 ± 0.7 | 3.4 ± 1.3 | 0.860 | 3.2 ± 0.6 |
| Albumin (g/dL) | 2.6 ± 0.2 | 2.7 ± 0.1 | 0.278 | 2.6 ± 0.1 |
| Ascites (%) | ||||
| • None or minimal | 65 | 69 | 0.284 | 67 |
| • Mild | 19 | 31 | 23 | |
| • Massive | 16 | 0 | 10 | |
| Esophageal varices (%) | ||||
| • None | 45 | 18 | 35 | |
| • Small | 33 | 55 | 0.336 | 41 |
| • Big/bad prognosis | 22 | 27 | 24 | |
| Survival (months) | 2.1 ± 0.5 | 5.5 ± 1.7 | 0.018 | 3.26 ± 0.7 |
Clinical characteristics of patients. αFP, Alpha fetoprotein.
| None (26) | Tamoxifen (13) | P | Total (39) | |
|---|---|---|---|---|
| Diagnoses (%) | ||||
| • Biopsy | 73 | 69 | 1.000 | 72 |
| • Alphafetoprotein and image | 27 | 31 | 28 | |
| Alphafetoprotein (ng/L) | 310 ± 74 | 533 ± 149 | 0.142 | 380 ± 70 |
| Alphafetoprotein (%) > 500 ng/L | 29 | 45 | 0.451 | 34 |
| Tumor size % | ||||
| • < 3 cm | 4 | 0 | 3 | |
| • 3-5 cm | 8 | 15 | 0.193 | 20 |
| • > 5 cm | 4 | 23 | 20 | |
| • Multinodular or diffuse | 84 | 62 | 77 | |
| Okuda (%) | ||||
| • I | 8 | 23 | 13 | |
| • II | 46 | 54 | 0.235 | 49 |
| • III | 46 | 23 | 38 | |
| Stage TNM (%) | ||||
| • I y II | 8 | 23 | 13 | |
| • III | 8 | 23 | 0.116 | 13 |
| • IV | 84 | 54 | 74 |
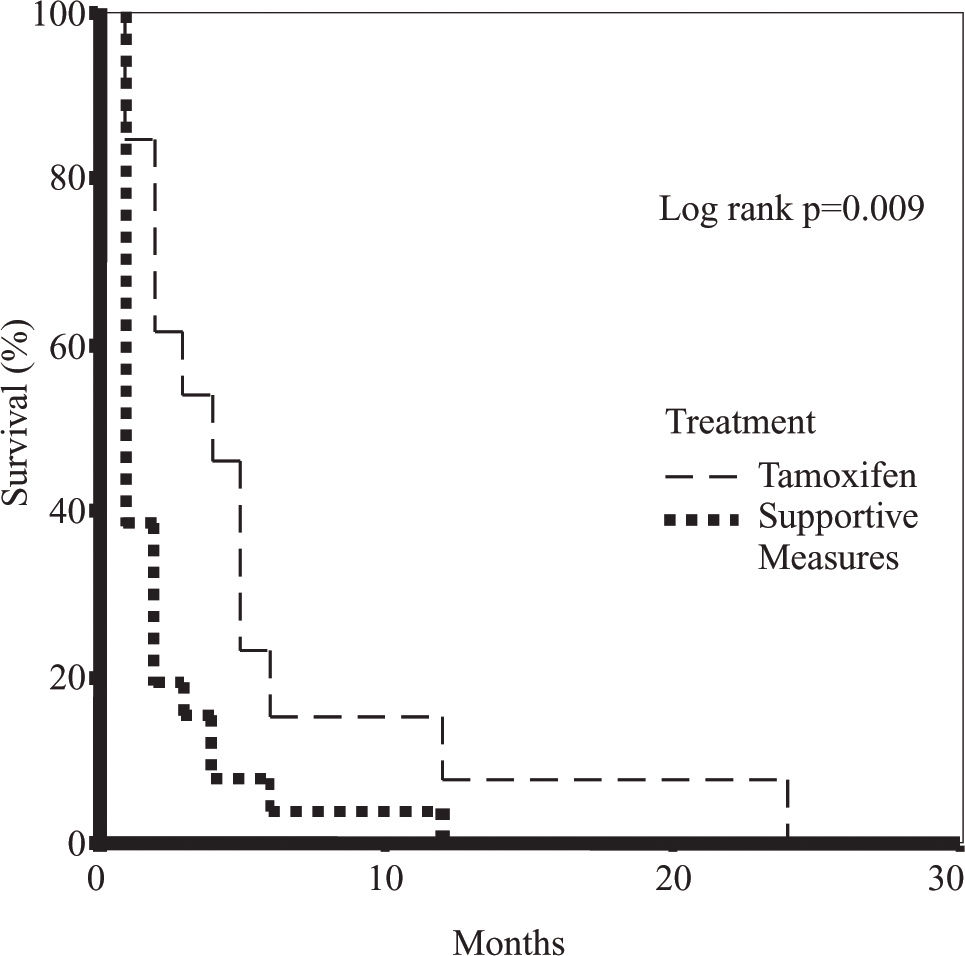
A greater survival was observed in the female gender (p = 0.038), in patients with a lower Okuda stage (p = 0.0149), and with serum bilirubin levels < 2.6 mg/dl (p=0.002) (Table III). Overall survival in the TMX-treated and the supportive measures groups was of 5.5 ± 1.7 months vs 2.1 ± 0.5 months, respectively (p = 0.018) (Figure 1). The multivariate analysis showed that treatment with TMX duplicates survival independently of the tumoral stage and hepatic reserve (p < 0.05).
Univariate survival analysis of the patients treated with tamoxifen compared with the supportive measures group.
| Variable | Mean survival (months) | P (log rank) | |
|---|---|---|---|
| Age | < 61 | 3.9 ± 1.2 | 0.302 |
| > 61 | 2.4 ± 0.6 | ||
| Sex | Male | 2.2 ± 0.6 | 0.038 |
| Female | 4.5 ± 1.3 | ||
| Cirrhosis | Present | 4.0 ± 1.2 | 0.204 |
| Absent | 2.4 ± 0.7 | ||
| Bilirubin | < 2.6 mg/dL | 4.5 ±1.1 | 0.002 |
| > 2.6 mg/dL | 1.5 ± 0.2 | ||
| Albumin | < 1.8 g/dL | 3.4 ± 0.8 | 0.635 |
| > 1.8 g/dL | 2.2 ± 0.9 | ||
| Alphafetoprotein | < 240 ng/L | 3.9 ± 1.3 | 0.649 |
| > 240 ng/L | 2.9 ± 0.8 | ||
| Ascites | |||
| • Low or absent | 2.9 ± 0.6 | ||
| • Moderate | 5.0 ± 2.5 | 0.173 | |
| • Massive | 1.3 ± 0.3 | ||
| Child-Pugh Score | |||
| • A | 4.0 ± 1.2 | ||
| • B | 3.2 ± 1.2 | 0.201 | |
| • C | 1.9 ± 0.5 | p = 0.122* | |
| TNM | |||
| • I y II | 6.8 ± 4.3 | ||
| • III | 3.4 ± 2.2 | 0.386 | |
| • IV | 2.6 ± 0.7 | ||
| Okuda | |||
| • I | 8.0 ± 4.5 | ||
| • II | 2.9 ± 0.6 | 0.201 | |
| • III | 2.0 ± 0.4 | p = 0.0149** | |
| Tamoxifen | • No | 2.1 ± 0.5 | 0.002 |
| • Yes | 5.5 ± 1.7 |
A 69 year old man with alcohol-related liver cirrhosis in a class A Child-Pugh score, presented with left-sided subcostal constant and sharp pain irradiated to the epigastrium. By the end of the same month the patient observed an epigastric painful mass of increasing size that reached a dimension of about 12 x 10 cm. The patient was referred to the oncology department of our institution; he had a weight-loss > 10 kg in two months and deterioration of liver function tests (ALT 127, AST 49, alkaline phosphatase 179). A computed tomography (CT) scan was performed and an irregular hypodense lesion image suggestive of HCC of 17 x 13.5 cm was found in the left lobe with areas of necrosis with calcifications within (Figure 2A). Also, the patient had alpha-fetoprotein levels of 350 ng/mL.
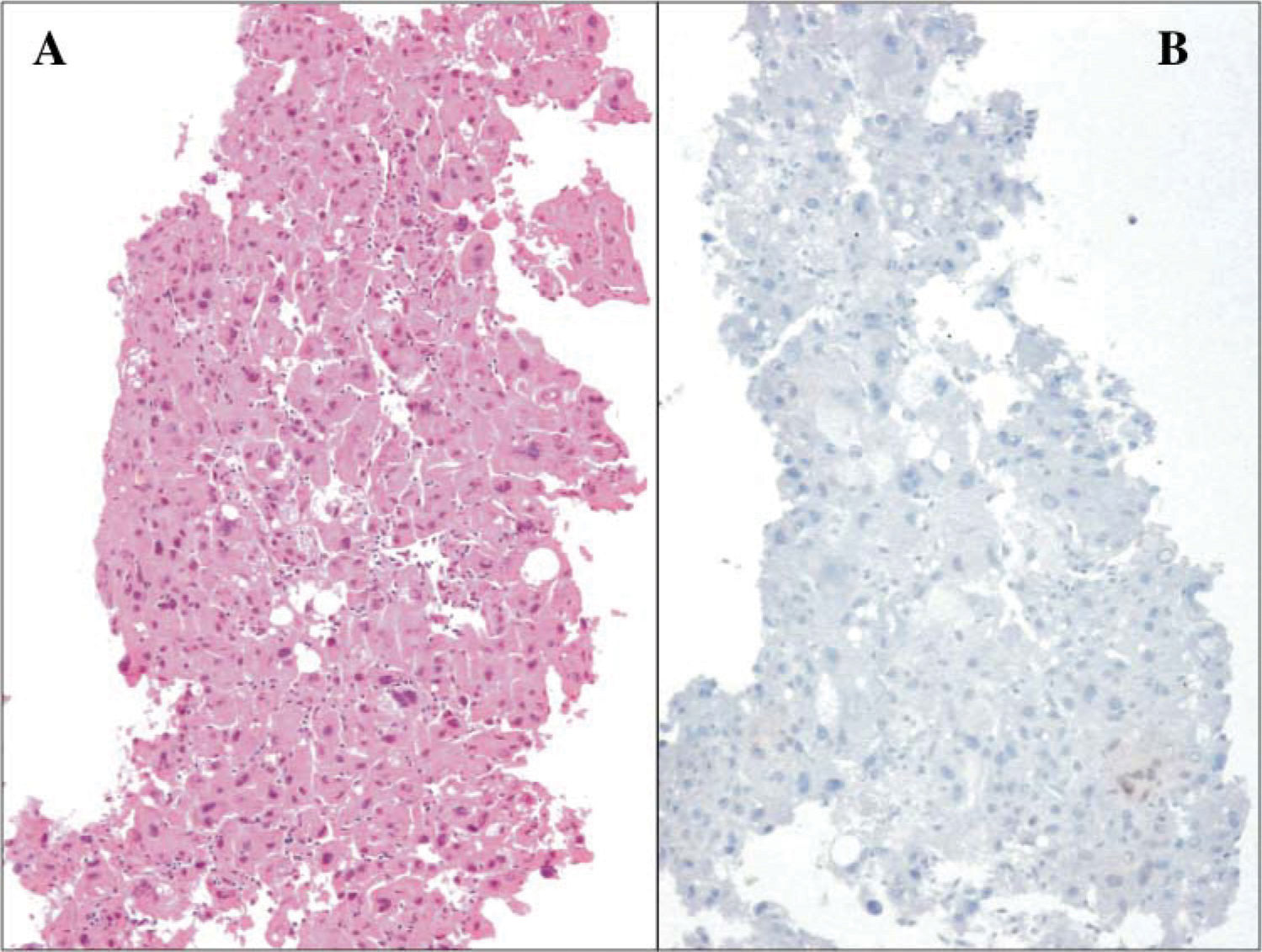
The lesion was biopsied and the diagnosis of moderately-differentiated hepatocellular carcinoma was made. The patient was not candidate to surgical treatment and started treatment with thalidomide during two months with progression of the disease (Figure 2B).
The patient then received TMX 40 mg/day and the next CT scan showed partial response 8 x 6 cm with a tumor reduction of 80% by the WHO criteria and the alpha-feto-protein levels were of 411 ng/dL (Figure 2C).
DiscussionDiverse hormonal treatments like TMX, anti-androgens, progestagens, luteinizing hormone antagonists and somatostatin, have been used.2 TMX is an estrogen receptor blocker with few secondary effects, lower cost when compared to other treatments, and of easy administration. TMX was introduced to clinical practice after three clinical trials that demonstrated an increase in overall survival with a dose of 20 to 30 mg/day.6,7,16 Nevertheless; these studies were carried out with a limited number of patients with HCC not suitable for loco-regional treatment due to a very advanced disease or a poor hepatic reserve.
Surgical, or other treatment modalities such as ethanol injection, intra-arterial embolization and systemic or intra-arterial chemotherapy, are not possible in a high percentage of patients. Chemotherapy has a limited activity, and in the only randomized study that compares anthracycline-based chemotherapy (with doxorubicin) to supportive measures alone shows an increase in overall survival (7.5 vs 10.6 weeks).15 Doxorubicin induces a partial response in only 3% of the patients, with a high frequency of fatal complications.
The CLIP Group did not show any benefits of the management with TMX vs supportive measures;14 however, they included patients with previous partial hepatectomy, orthotopic liver transplant, ethanol injections, intra-arterial embolizations, and systemic or intra-arterial chemotherapy in 47% of cases; therefore with a very different population to that of our study. This study contained patients with HCC at more advanced stages at the moment of diagnosis without the possibility of surgical resection. The mean survival was of 16 and 15 months in the TMX and supportive measures groups, respectively; a very prolonged survival when compared to that of patients with unresectable HCC of other series including ours (mean = 8 weeks). Castells et al in a randomized trial did not find a benefit in overall survival with a TMX dose of 20 mg; nevertheless, in this study patients with terminal disease were excluded, differently to our study.12 Other two studies have not found a benefit in overall survival with the use of TMX.13,17 Another phase III clinical trial included 200 patients in the TMX-treated group and 210 were assigned to the control group. Patients and tumor characteristics were well balanced between both groups. Most patients were men with underlying alcoholic cirrhosis. Previous treatments such as surgery, chemoembolization, percutaneous ethanol injection, or systemic chemotherapy were performed in 61 patients (14.5%). Outcome did not differ between the two treatment arms: estimated median survival was 4.8 and 4.0 months in the TMX and in the control groups, respectively.18
The patients in our study received low dosages of tamoxifen (20-40 mg/day). There are studies that compare high tamoxifen dosages (120 mg/day) vs intermediate dosages (60 mg/day) without showing any differences regarding response or survival, but with a greater frequency of adverse effects (Chow 2002). We did not find any significant adverse effects secondary to the administration of tamoxifen at the dosages employed.
On the other hand, there are multiple reports of tumoral regression secondary to the use of TMX, as well as randomized studies of the use of TMX with luteinizing hormone inhibitors that show an increase in overall survival of 282 vs 127 days when compared to placebo.21
It is important to mention that the expression of estrogen receptors has not been related to the response to TMX as in the patient with a partial response with a 40 mg/ dose and negative estrogen receptors (Figure 3).17 TMX has been widely used in neoplastic diseases like breast and endometrial cancer. It is thought that TMX has several antineoplastic mechanisms; one of them is cellular replication inhibition due to its competitive union to the estrogen receptor.21 Other antineoplastic effects of TMX are through its interference with calmodulin to inhibit protein kinase C, growth inhibition and apoptosis induction in cultured cells that do not express estrogen receptors.16 Additionally, TMX reduces the production of Insulin like growth factor in HCC cells. As for breast cancer, a variant form of the estrogen receptor (ER) alpha transcript has been described in HCC. It is derived by an exon 5-deleted transcript (vER), which lacks the hormone-binding domain of the receptor but, being intact in the DNA-binding domain, maintains constitutive transcriptional activity. HCC presenting vER has an extremely aggressive clinical course and is unresponsive to antiestrogen therapy.21
Histopathological analysis (A) Hematoxilin-eosin stain showing neoplastic epithelial cells forming thick strings with nuclear atypia and abundant cytoplasm (B) Immunohistochemical analysis of liver biopsy of a patient with partial response showing negative immunoreactivity (High magnification 200 x).
The benefits of the treatment with tamoxifen are limited and do not seem to be associated to the presence of estrogen receptors. Studies must be directed to determine pathological and molecular factors that could predict the tumoral response or a prolonged stable disease secondary to the administration of this drug.