Obesity and related disorders are a common cause of morbidity worldwide. Nonalcoholic fatty liver disease is the most important hepatic consequence of adipose accumulation. There is strong evidence of obesity-related disorders as risk factors for hepatocellular carcinoma and of nonalcoholic fatty liver disease as a cause of hepatocellular carcinoma, but it is apparently less important than other chronic liver diseases. Unfortunately, preventive measures are not well validated in the population of patients with NAFLD. In this review, we analyze the available information supporting the increased risk of hepatocellular carcinoma in obese patients and patients with nonalcoholic fatty liver disease, considering the epidemiological and basic research-derived evidence.
Hepatocellular carcinoma (HCC) is an important cause of death worldwide.1-4 According to the World Health Organization, it was responsible for 618,124 deaths worldwide during 2002, and although it is the third greatest cause of cancer-related death, it is not among the first 20 general causes of death. However, during the next few years, this scenario will tend to worsen, as liver cancer becomes the 13th cause of general death worldwide, responsible for 1.4% of global deaths.5
The etiology of HCC differs according to geographic, economic, and health status, most commonly involving alcohol6 and hepatitis C and hepatitis B virus infections.2 However, other factors are related to this cancer, including exposure to biological7 and chemical substances (e.g., aflatoxins and polyvinyl chloride, respectively).8
Other less common risk factors have been associated with HCC, such as tobacco smoking, type 2 diabetes mellitus, and obesity.9,10 These risk factors are very important, because today and in the near future, their incidence will continue to increase globally.11
Nonalcoholic fatty liver disease (NAFLD) is one of the systemic consequences of adipose tissue accumulation and is a common cause of altered liver function.12 The available information regarding the natural history of NAFLD indicates that it could progress to end-stage liver disease and related complications, including HCC.13 Because this is a young disease in terms of its first description,14 information about the role of NAFLD as an important cause of HCC is scarce.
The aim of this review it is to examine the experimental and clinical information about the role of NAFLD and related disorders (mainly those derived from metabolic syndrome) in the pathogenesis of HCC.
Diabetes mellitus and obesityMore than 20 years ago, the initial epidemiological association between diabetes mellitus and HCC was de-scribed as an epiphenomenon rather than a risk factor.15 This relationship was not initially confirmed in small studies.16 However, with more robust epidemiological evidence, it was clear that diabetes mellitus significantly increases the risk for HCC (odds ratio [OR] 3.3-18.5).17 This finding was confirmed in huge samples in USA and non-USA populations,18,19 pointing to diabetes mellitus as an independent factor20 or cofactor21-23 in the development of HCC. Diabetes mellitus is not associated with HCC only, but worse prognoses were observed in pa-tients with HCC and diabetes mellitus than in patients with HCC without diabetes mellitus.24
Several hypotheses suggest an etiological role for diabetes mellitus in the pathogenesis of HCC. Insulin and its precursors, pro-and pre-proinsulin, have been shown to have some homology to the insulin-like growth factors. Moreover, they have some affinity to receptors of the tumor growth factors. Therefore, an association between diabetes mellitus, insulin, hyperinsulinemia, and carcino-genesis appears plausible.25 Several lines of experimental evidence have suggested a putative mechanism by which diabetes-related carcinogenesis is induced. In an interesting animal model used to test the influence of hyperg-lycemia and hyperinsulinemia, high levels of insulin and glucose, together with high glucokinase expression, led to the upregulated expression of the Fas gene, possibly mediated by the mitogen-activated protein kinase (MAPK) pathway. Fas encodes a multifunctional enzyme complex that catalyzes different reactions in the biosynthesis of long-chain fatty acids, starting from acetyl-CoA and malonyl-CoA, which is overexpressed in several neo-plasms.26 Unfortunately, few in vivo models are available in which to study this phenomenon. An outbred mouse model of type 2 diabetes mellitus that develops HCC spontaneously was recently described, which could be an interesting research tool.27
Obesity is also associated with an increased risk of HCC in subjects with well-documented chronic liver diseases and also in patients with cryptogenic cirrhosis, so that obesity is an independent risk factor for HCC (OR = 1.9, 95% confidence interval [CI] 0.9-3.9).28-29 This increased risk is apparently mediated by the presence of cryptogenic cirrhosis. The results of an analysis of 30,201 adult liver transplantation recipients demonstrated that in this cohort, the most common cause of liver transplantation was hepatitis C virus infection, but in obese patients, the most common cause of liver transplantation was cryptogenic cirrhosis, indicating the role of this relationship. In the same study, a higher incidence of HCC was observed with increasing body mass index. It was concluded that obesity was an independent risk factor for HCC in this cohort (OR = 1.65; 95% CI, 1.222.22; P = 0.001) but only in subjects with alcoholic cirrhosis or cryptogenic cirrhosis.30
No difference was observed in the frequency of HCC in subjects with obesity-related cryptogenic cirrhosis and matched patients with hepatitis C virus-infection-related cirrhosis (30 vs 21%, respectively), which suggests similar carcinogenic effects.31
There has been special interest in the study of leptin and HCC to explain the observed epidemiological association. Leptin is produced by adipocytes and plays an important role in the control of food intake and energy metabolism.32,33 Leptin is a 16-kDa protein secreted from the adipose tissue. Since the discovery of leptin, our un-derstanding of its physiological role has evolved from that of a satiety signal to that of an integrative hormone that responds to and regulates different endocrine pathways with direct metabolic effects on peripheral tissues. It has been suggested that leptin also plays a role in the development of HCC. It has been demonstrated in human studies that serum leptin levels are higher in subjects with cirrhosis, even when they are not obese, but are not associated with an increased risk of HCC.34 However, these findings were not confirmed in another clinical study,35 so nutritional factors could account for these discrepancies.
Similarly, one study that analyzed the expression of the leptin receptor (Ob-R) in pathologically confirmed HCC showed confused results, which suggests that high expression of Ob-R is associated with a higher microvas-cular density in tumors but with a low risk of HCC and a better survival rate.36 This is consistent with a murine study in which leptin administration resulted in the significant inhibition of HCC growth and improved the survival rate in athymic mice. This can be explained by the relative expansion of the natural killer cell population induced by the administration of leptin, because leptin induced a dose-dependent increase in natural killer cyto-toxicity in vitro,37 although this did not occur in another study.38
Conversely, several in vitro studies have shown that leptin increases cellular proliferation and reduces apop-tosis.39,40 Even more confusing is that in vitro evidence suggests that leptin promotes HCC growth, invasiveness, and migration.41 The available information about the role of leptin in HCC is insufficient to define a specific role for it in the development of HCC. It is possible that other endocrine and paracrine variables are involved.42
Nonalcoholic fatty liver diseaseThe information derived from epidemiological studies indicates that HCC is one of the liver-related causes of death in subjects with NAFLD. The results of the Rochester Epidemiology Project, which included subjects with biopsy-proven (17%) and without biopsy-proven NAFLD (83%), reported malignancies (not including liver malignancy) as the main cause of death, followed by ischemic heart disease, and liver-related disease as the third most important cause of death. HCC is the third greatest cause of all liver-related deaths, responsible for 0.5% of overall deaths. However, this proportion increases considerably in subjects with cirrhosis, suggesting that advanced fibrosis is a risk factor for developing HCC.43 Similarly, in a Japanese study that included 247 patients with biopsy-proven NAFLD, only five patients (2%) developed HCC during 44 months of follow-up, although all these patients were in the group with advanced fibrosis. The five-year cumulative incidence of HCC was 20%.44 Based on this evidence, only those patients with NAFLD and advanced fibrosis should be screened for HCC, but until now, there has been no cost-benefit analysis of such screening. This could explain why subjects with crypto-genic cirrhosis receive are less frequently screened for HCC, with important effects on therapy.45
The natural history of HCC in subjects with NAFLD and advanced fibrosis is the same as that observed in subjects with other chronic liver diseases and the same grade of fibrosis,46 and almost all NAFLD patients with HCC also present with liver cirrhosis. However, liver cirrhosis is not present in all cases. In a small series of HCC in subjects with NALFD, cirrhosis was not observed in ~30-40% of cases.47 Fortunately, in NAFLD patients with nonad-vanced liver disease and HCC, the long-term prognosis could be better than in patients with other etiologies.48
An important phenomenon must be considered before any analysis is made of a patient with HCC and a putative NAFLD-related etiology, referred to as “born-out”. In this phenomenon, most of the histological hallmarks of nonalcoholic steatohepatitis (NASH) disappear when the disease progresses to cirrhosis. This phenomenon can explain why non-B and non-C hepatitis virus infections occurred in 29.8% of a cohort of patients with HCC, whereas only 2.4% were associated with NASH.49 Similar trends have been confirmed in other cohorts.45 However, we must keep in mind that fatty changes were observed in up to 20% of all patients with HCC.50 When a cohort of patients with HCC was analyzed, cryptogenic cirrhosis caused 6.9% of cases, a less important cause of HCC after hepatitis C virus infection (54.9%), hepatitis B virus infection (16.2%), and alcohol-related HCC (12.9%). The main characteristics of metabolic syndrome were observed more frequently in patients with cryptogenic cirrhosis than in those with other chronic liver diseases.45,52
It is important to note the role of steatosis and metabolic syndrome separately of NAFLD. The role of NAFLD in the natural history of patients infected with hepatitis C virus is well recognized,52,53 but little information about its role in HCC is available. Liver steatosis apparently increases the risk of HCC and is an independent risk factor even more important than age or other common risk factors. This risk could be as high as sixfold,54 even in patients with a treated and sustained virological response.55
As mentioned previously, those subjects with NAFLD apparently receive less frequent ultrasound screening for HCC. Today, the screening programs that could be performed in NAFLD patients are deduced from data concerning other chronic liver diseases. Little information originating from NAFLD patients is available. Beal et al.56 retrospectively analyzed the reliability of serum biomarkers for HCC in subjects with NAFLD relative to that in patients with other chronic liver diseases, using prothrombin induced by an absence of vitamin K, glypi-can proteoglycan 3, squamous cell carcinoma antigen 1, and follistatin. The authors demonstrated a higher accuracy in detecting HCC compared with the accuracy a-fe-toprotein alone, particularly in subjects with alcoholic liver disease/NAFLD. Unfortunately, data comparing pro-spective vs ultrasound screening (standard of care) are not yet available.
Experimental evidenceAnimal models of HCC are scarce, and a long waiting period is required to observe the relevant events in these models. There are many interesting models in the field of NAFLD. One uses a genetically obese, leptin-deficient ob/ob mouse, which shows increased contents of phos-phorylated MAPK isoforms (p42 Erk-1 and p44 Erk-2), suggesting a more intense prereplicative phase of hepa-tocyte proliferation. These changes are consistent with the important increase in the hyperdiploid DNA content, indicating a significant proliferative state. Interestingly, these events do not appear to be related to increased hepatocyte apoptosis, suggesting that one or more of the cellular mechanisms that inhibit apoptosis are induced in ob/ob livers, such as NF-KB, MnSOD, Bfl-1, and Bcl-XL. All these data suggest that hepatocyte hyperplasia occurs in this animal model of NAFLD.57
The second animal-based evidence comes from C57BL/6J male mice on a choline-deficient, L-amino-acid-defined diet. In these animals, fatty liver and fibro-sis, but not cirrhosis, were observed after 84 weeks, with hepatocellular adenomas present in 66.7% of mice and HCC in 20.8% of mice. These neoplastic liver changes were not observed in female mice.58
Another model is derived from a transgenic mouse expressing the dominant negative form of the retinoic acid receptor, RAR. This mouse cannot bind retinoic acid and inhibits the binding of the normal RAR/RXR het-erodimer to the retinoic-acid-responsive element of target genes. In this transgenic mouse, an important reduction in mitochondrial /3-oxidation, with a concomitant increase in peroxisomal /3-oxidation and microsomal «-oxi-dation, leads to hepatic steatosis in nonobese mice, with important focal necrosis. Liver tumors are developed by 50% of mice after 12 months, and by 80% of mice after 18 months. These neoplastic events could be related to the increased activation of peroxisome proliferator-acti-vated receptor (PPAR)a and PPAR3.59
In a similar way, a knockout mouse for the gene that encodes the enzyme predominantly responsible for the catabolism of excess hepatic S-adenosylmethionine, glycine N-methyltransferase, developed steatosis and fibrosis at three months of age, which was more pronounced at eight months of age. In this period of their life, all these mice developed multifocal HCC. The putative mechanism involved suggests the diminished expression of Ras and JAK/STAT inhibitors, and the increased expression of antiapoptotic proteins, such as Bcl-XL, which promote abnormal proliferation. Epige-netic changes consequent upon the increased S-adeno-sylmethionine/S-adenosylhomocysteine ratio promoted aberrant methylation.60 Similar histological chang-es were observed in 25-month-old galectin-3 knockout mice.61
In the fatty liver Shionogi mouse, a nonobese mouse with severe fatty liver, the incidence of HCC is 40 and 9.5% in males and females, respectively, at 16 months. This mouse develops HCC without developing liver cirrhosis.62
These data suggest an important antiapoptotic and proliferative state in the animal models of NAFLD, which could explain the association between NALFD and HCC.
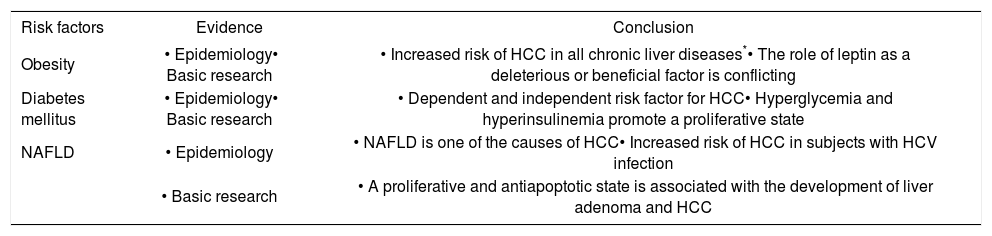
ConclusionIn the near future, HCC will become an important cause of death, and concomitantly, obesity and related disorders (such as NAFLD) will be important causes of morbidity. NAFLD is a cause of HCC, probably with lower relevance than other prevalent etiologies of chronic liver diseases. However, at the moment, there is no high-quality information about the long-term consequences of NAFLD or proper preventive measures available to these patients. All the information from experimental models should be useful to clarify these uncertainties (Table I).
Obesity and related disorders as risk factors for hepatocellular carcinoma.
| Risk factors | Evidence | Conclusion |
|---|---|---|
| Obesity | • Epidemiology• Basic research | • Increased risk of HCC in all chronic liver diseases*• The role of leptin as a deleterious or beneficial factor is conflicting |
| Diabetes mellitus | • Epidemiology• Basic research | • Dependent and independent risk factor for HCC• Hyperglycemia and hyperinsulinemia promote a proliferative state |
| NAFLD | • Epidemiology | • NAFLD is one of the causes of HCC• Increased risk of HCC in subjects with HCV infection |
| • Basic research | • A proliferative and antiapoptotic state is associated with the development of liver adenoma and HCC |