Orthotopic liver transplantation (OLT) can be associated with significant bleeding requiring multiple blood product transfusions. Rotational thromboelastometry (ROTEM) is a point-of-care device that has been used to monitor coagulation during OLT. Whether it reduces blood loss/transfusions during OLT remains controversial.
Materials and MethodsWe aim to compare ROTEM with conventional coagulation tests (aPTT, PT, INR, platelet count, fibrinogen) to guide transfusion of platelets, cryoprecip-itate, and fresh frozen plasma (FFP) during OLT over 3 years. Thirty-four patients who had transfusions guided by ROTEM were compared to 34 controls who received transfusions guided by conventional coagulation tests (CCT). Intraoperative blood loss, type/ amount of blood products transfused, and direct costs were compared between the two groups.
ResultsThe ROTEM group had significantly less intra-operative blood loss (2.0 vs. 3.0 L, p = 0.04) and fresh frozen plasma (FFP) transfusion (4 units vs. 6.5 units, p = 0.015) compared to the CCT group (2.0L vs. 3.0L, p = 0.04). However, total number of patients transfused cryoprecipitate was increased in ROTEM (n = 25;73%) as compared to CCT (n = 19; 56%), p = 0.033. The direct cost of blood products plus testing was reduced in the ROTEM group ($113,142.89 vs. $127,814.77). Conclusion. In conclusion implementation of a ROTEM-guided transfusion algorithm resulted in a reduction in intra-operative blood loss, FFP transfusion and a decrease in direct cost during OLT. ROTEM is a useful and safe point of care device in OLT setting.
Orthotopic liver transplantation (OLT) can be associated with significant bleeding requiring transfusion of multiple blood products, especially in patients with advanced liver disease. Conventional coagulation tests (CCT) such as prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen, and platelet count are used to guide transfusions in liver transplant setting. However, these tests are limited by their inability to assess clot strength and the functionality of fibrinogen or hyperfibrinolysis that may be present. Rotational thromboelastometry (RO-TEM) monitors viscoelastic properties of whole blood clot formation and degradation, allowing for a comprehensive view of the entire coagulation cascade. Rotational thromboelastometry (ROTEM) is a point-of-care device that has been successfully used to monitor coagulation on whole blood samples during cardiac surgery and trauma.1-4 Literature on its use in the setting of OLT is growing.5-7
In patients undergoing liver transplantation, ROTEM has been shown to quickly and correctly predict hypofi-brinogenemia, thrombocytopenia, and decreased clot firmness.8 Furthermore, researchers suggest that while PT and INR are used as prognostic indicators and to guide blood product management, these tests are poor predictors of bleeding risk in liver disease.9 This challenges the current practice of correcting an abnormal INR with fresh frozen plasma (FFP) prior to invasive procedures, such as OLT. Overall bleeding risk cannot be accurately predicted by INR, as it measures only one of the many components of coagulation and clot stability. Transfusion of pRBCs, FFP, and platelets has been shown to be associated with increased morbidity and mortality in transplantation as well as worse graft survival.2
In addition to increased rates of infection and prolonged stays in intensive care units,3 increased transfusion rates can be very costly to both the patient and hospital. Toner, et al. found in their cross-sectional randomized study of 213 US centers that the mean cost for one unit of pRBCs was $210.74 ± 37.9 and the mean charge to the patient was $346.63 ± 135.10
Few studies have compared the cost of blood products and coagulation factors before and after the implementation of a ROTEM protocol. Gorlinger, et al. found an overall savings of 36% after implementation of ROTEM in visceral and liver transplantation surgery in Germany. Reduction in the cost of blood products with increase in cost of coagulation factors resulted in a decrease in overall cost.7 Cost reduction with the use of ROTEM has also been reported in cardiac and neurosurgery.11,12
Our primary aim in this study was to assess the impact of a ROTEM-guided transfusion protocol on intra-opera-tive blood loss during OLT. We also assessed intra-operative transfusion requirements (total and for each blood component) and the direct cost of ROTEM based & conventional coagulation tests and blood products.
Materials and MethodsSixty-eight consecutive patients who underwent OLT from 2012 to 2014 at The Ohio State University Wexner Medical Center (OSUWMC) were included in this study. Data of thirty-four patients who received ROTEM guided transfusions after July 1, 2013 was collected prospectively and compared to the prior thirty-four patients who received transfusions based on conventional coagulation tests (PT, aPTT, INR, hemoglobin, platelets, and fibrinogen). There was no randomization and standardized anesthesia and surgical techniques were used; surgeries were performed by three equally experienced surgeons. Hemodynamic monitoring, antibiotics and immunosuppressive medications were also standardized in the two groups. All patients age 18 years or older who received a liver graft from a deceased donor and underwent OLT were included. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki reflected in an exemption granted by our hospital Institutional Review Board.
Intra-operative blood product use (type and amount) as well as coagulation test results pre- and post-OLT were collected on all patients. Blood products given during OLT were pRBCs, FFP, cryoprecipitate, platelets, and salvaged blood. The amount of salvaged blood, which was recorded in milliliters, was converted to units by dividing the volume by 200 and then multiplying by 55%. This was based on one unit of pRBC having a red cell mass of 200 mL and salvaged blood having an approximate hematocrit of 55%, which has been previously reported.13 Salvaged blood was converted to units so the total amount of blood transfused could be calculated in units. Intra-operative blood loss was estimated and recorded by the anesthesia provider. Data was extracted from charts in the electronic medical record, de-identified, and used to construct a database of OLT recipients with information on risk factors, pre-operative, and post-operative testing.
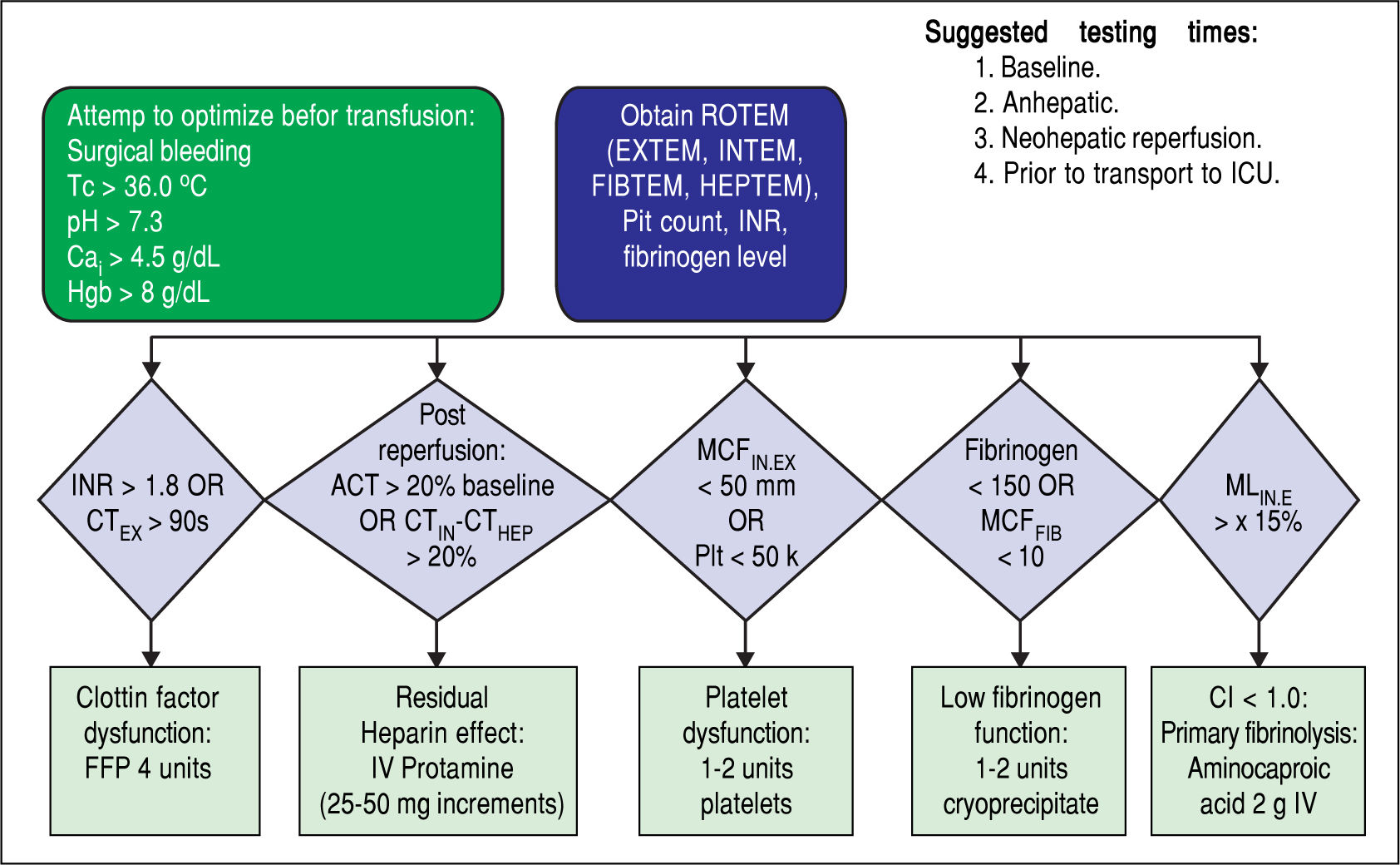
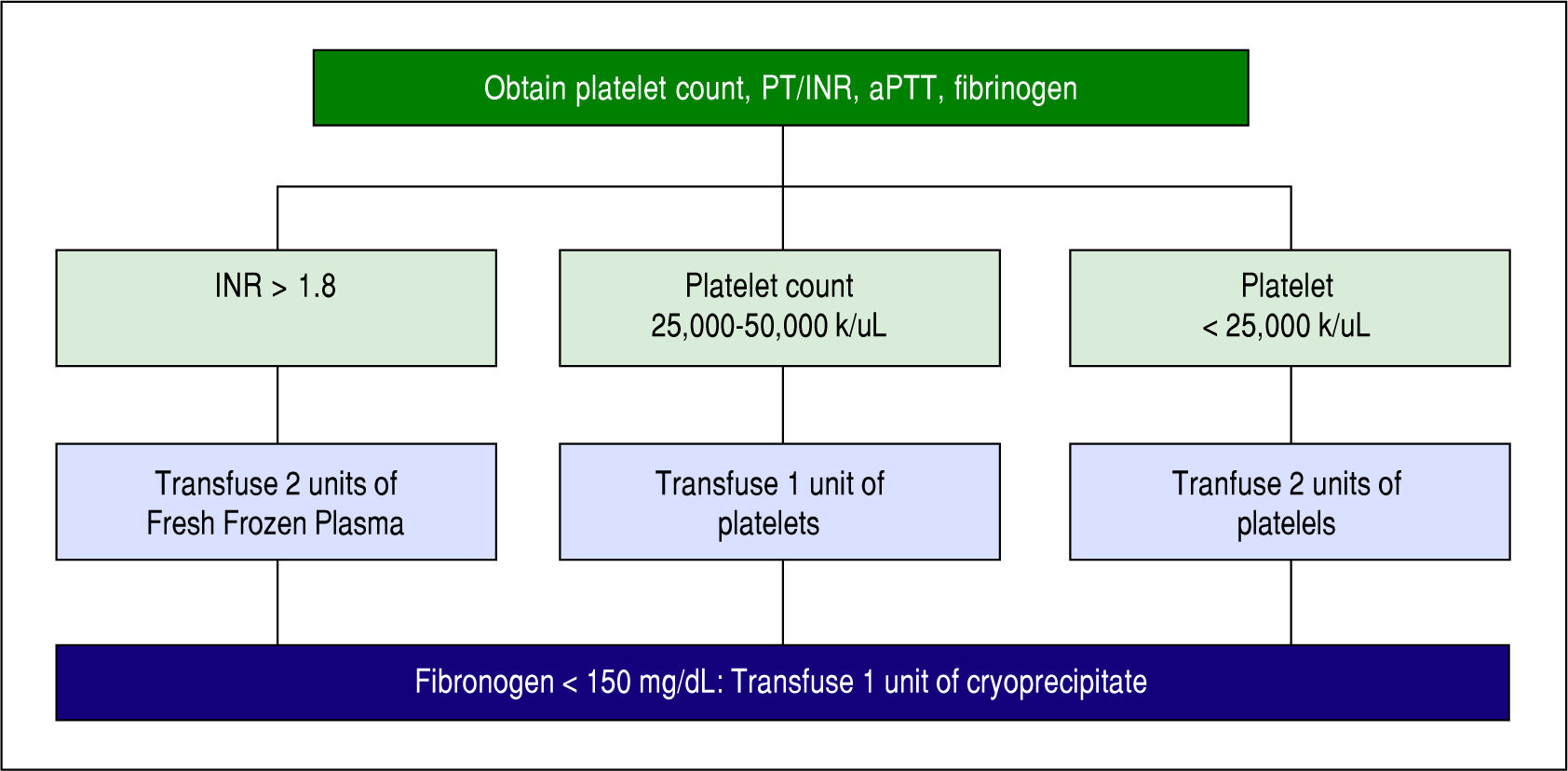
Blood products were transfused in the ROTEM group based on the algorithm in Figure 1. Four assays (EXTEM, INTEM, FIBTEM, and HEPTEM) were completed for each patient in the ROTEM group after optimizing calcium, temperature, pH, and hemoglobin. In EXTEM, coagulation is activated by a small amount of tissue thromboplastin and allows for assessment of factors VII, X, V, II, I, platelets, and fibrinolysis. In INTEM, coagulation is activated via the contact phase and is sensitive for factor deficiencies of the intrinsic coagulation cascade (factors XII, XI, IX, VIII, X, V, II, I) and for the presence of heparin in the sample. In FIBTEM, coagulation is activated as in EXTEM but cytochalasin D is added so platelets are blocked. The resulting clot is therefore only dependent on fibrin formation and polymerization so fibrin deficiencies are detected. In HEPTEM, coagulation is activated as in INTEM but heparinase in the reagent degrades heparin present in the sample so abnormalities due only to heparin are detected. Based on these assays, FFP, platelets, and/or cryoprecipitate were transfused according to our algorithm in Figure 1. Protamine or aminocaproic acid were also given based on the ROTEM results for residual heparin effect or primary fibrinolysis, respectively. Unfortunately, number of patients given either of these was not recorded in this study. Prior studies have shown that 20-36% of patients undergoing OLT develop hyperfi-brinolysis and aminocaproic acid is a treatment.6,14,15 Blood products including platelets and FFPs were transfused in the CCT group per the protocol in Figure 2, and fibrinogen was transfused only if the patient was still bleeding or fibrinogen was still low.
During OLT temperature was maintained above 36 °C, and pH was kept above 7.3. Calcium was infused to keep the ionized calcium level above 4.5 g/dL and hemoglobin was maintained above 8 g/dL. ROTEM tests (EXTEM, INTEM, FIBTEM, and HEPTEM) were only performed for patients in the ROTEM group according to the manufacturer's instructions by anesthesia providers. Coagulation tests for all patients and ROTEM tests for patients in the ROTEM group were performed at baseline, at the an-hepatic phase, at neo-hepatic reperfusion, prior to transfer to the intensive care unit, and at any other time deemed necessary by the clinical judgement of the anesthesia provider or surgeon.
The direct cost per unit of each type of blood product was obtained from OSUWMC's Transfusion Services, and total cost of products was then calculated. The cost of conventional coagulation tests was obtained from OSUW-MC's laboratory services, and the cost of ROTEM reagents was obtained from Tem Systems, Inc. The cost of operating the ROTEM (manpower) is equivalent to performing the laboratory tests so this cost was not included. The total costs for testing were then calculated based on running four ROTEMs or four sets of conventional coagulation tests per surgery, which is the average number of tests performed during a liver transplant at OSUWMC.
Our sample size of sixty-eight patients was based on a pilot project at OSUWMC that evaluated a total of sixteen patients. The amount of blood products transfused and in-traoperative blood loss were studied in the pilot project, with a trend toward decreased total blood product use (10.1 units vs. 20.5 units) and blood loss (2.0 units vs. 3.0 units) found in the ROTEM group. With the χ2 test and a 2-sided α value of 0.05 as the significance criterion, a sample size of thirty-four patients per group was calculated to provide 80% power to demonstrate the influence of RO-TEM on decreasing intraoperative blood loss during OLT.
Baseline patient characteristics as well as pre- and post-procedure laboratory values were compared between patients transfused using the ROTEM algorithm and CCT. Fisher exact tests were used to test for significant differences between the ROTEM and CCT groups with discrete variables such as race and gender. With continuous variables that were approximately normally distributed (BMI, MELD, and age), two-sample t-tests were used to test for significant differences between groups. Due to the non-normality of all laboratory values and ICU length of stay, nonparametric Wilcoxon rank-sum tests were used to test for significance.
Blood product usage and blood loss during OLT comparing ROTEM vs. CCT guided transfusion were also analyzed. Due to the non-normality of the data, nonpara-metric Wilcoxon rank-sum tests were used to test for significant differences between the two groups in terms of the use of the 5 different blood products and blood loss. The sum of pRBCs, FFP, platelets, cryoprecipitate, and salvaged blood in units was used as an indicator of total blood product use. Results are expressed as medians and interquartile ranges. A two-tailed P value < 0.05 was considered significant.
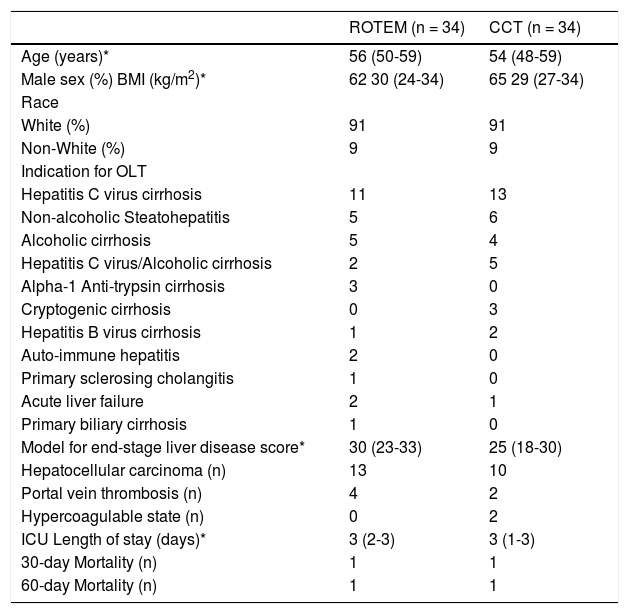
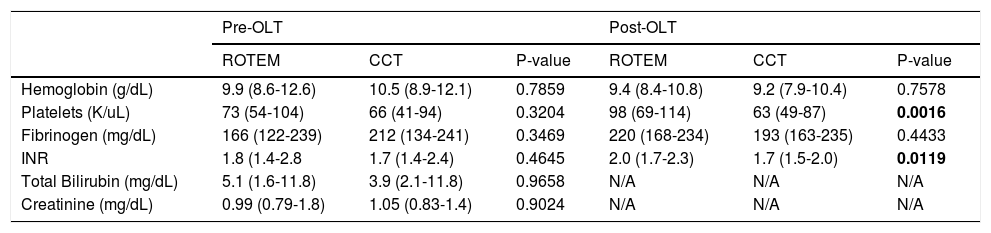
ResultsFrom May 2012 to December 2014, 68 consecutive patients who underwent OLT were included: 34 patients each in the ROTEM group and the CCT group. Patient characteristics were comparable between groups as shown in table 1. The reported MELD scores are calculated from laboratory data and exclude any exception points. Laboratory values prior to and immediately post OLT are presented in table 2. No significant differences were observed among the baseline laboratory values between groups. After surgery, both the INR and platelet count were significantly higher in the ROTEM group (2.0 vs. 1.7, p = 0.01 and 98000 vs. 63000, p = 0.002, respectively).
Patient characteristics.
| ROTEM (n = 34) | CCT (n = 34) | |
|---|---|---|
| Age (years)* | 56 (50-59) | 54 (48-59) |
| Male sex (%) BMI (kg/m2)* | 62 30 (24-34) | 65 29 (27-34) |
| Race | ||
| White (%) | 91 | 91 |
| Non-White (%) | 9 | 9 |
| Indication for OLT | ||
| Hepatitis C virus cirrhosis | 11 | 13 |
| Non-alcoholic Steatohepatitis | 5 | 6 |
| Alcoholic cirrhosis | 5 | 4 |
| Hepatitis C virus/Alcoholic cirrhosis | 2 | 5 |
| Alpha-1 Anti-trypsin cirrhosis | 3 | 0 |
| Cryptogenic cirrhosis | 0 | 3 |
| Hepatitis B virus cirrhosis | 1 | 2 |
| Auto-immune hepatitis | 2 | 0 |
| Primary sclerosing cholangitis | 1 | 0 |
| Acute liver failure | 2 | 1 |
| Primary biliary cirrhosis | 1 | 0 |
| Model for end-stage liver disease score* | 30 (23-33) | 25 (18-30) |
| Hepatocellular carcinoma (n) | 13 | 10 |
| Portal vein thrombosis (n) | 4 | 2 |
| Hypercoagulable state (n) | 0 | 2 |
| ICU Length of stay (days)* | 3 (2-3) | 3 (1-3) |
| 30-day Mortality (n) | 1 | 1 |
| 60-day Mortality (n) | 1 | 1 |
No difference was statistically significant. * The data is expressed as medians and interquartile ranges.
Laboratory values pre- and post-OLT in two intervention groups.
| Pre-OLT | Post-OLT | |||||
|---|---|---|---|---|---|---|
| ROTEM | CCT | P-value | ROTEM | CCT | P-value | |
| Hemoglobin (g/dL) | 9.9 (8.6-12.6) | 10.5 (8.9-12.1) | 0.7859 | 9.4 (8.4-10.8) | 9.2 (7.9-10.4) | 0.7578 |
| Platelets (K/uL) | 73 (54-104) | 66 (41-94) | 0.3204 | 98 (69-114) | 63 (49-87) | 0.0016 |
| Fibrinogen (mg/dL) | 166 (122-239) | 212 (134-241) | 0.3469 | 220 (168-234) | 193 (163-235) | 0.4433 |
| INR | 1.8 (1.4-2.8 | 1.7 (1.4-2.4) | 0.4645 | 2.0 (1.7-2.3) | 1.7 (1.5-2.0) | 0.0119 |
| Total Bilirubin (mg/dL) | 5.1 (1.6-11.8) | 3.9 (2.1-11.8) | 0.9658 | N/A | N/A | N/A |
| Creatinine (mg/dL) | 0.99 (0.79-1.8) | 1.05 (0.83-1.4) | 0.9024 | N/A | N/A | N/A |
The data is expressed as medians and interquartile ranges.
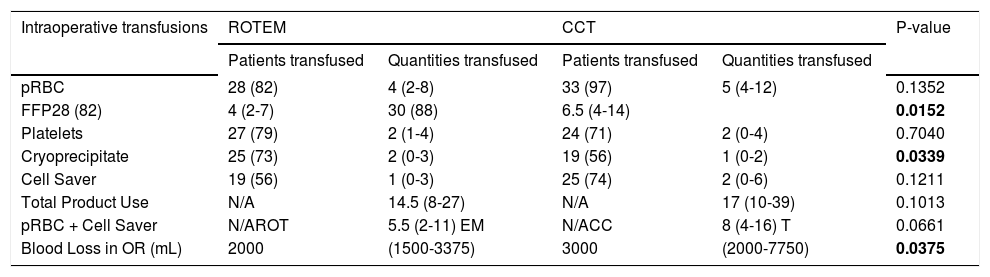
The number of patients transfused each blood product is presented in table 3, along with the subsequent amount of each product transfused. Both individual blood products and the total of all blood products were compared between groups. Patients in the ROTEM group received significantly less FFP (4 units vs. 6.5 units, p = 0.02) but more cryoprecipitate (2 units vs. 1 units, p = 0.04). The total amount of blood products transfused was less in the ROTEM group but did not reach statistical significance (14.5 units vs. 17 units, p = 0.11). The amount of blood transfused (pRBCs + salvaged blood) was also less in the ROTEM group (5.5 units vs. 8 units, p = 0.07). Intraoperative blood loss was significantly lower in in the ROTEM group (2.0 vs. 3.0 L, p = 0.04).
Number of patients and quantities of transfusions of blood products during OLT.
| Intraoperative transfusions | ROTEM | CCT | P-value | ||
|---|---|---|---|---|---|
| Patients transfused | Quantities transfused | Patients transfused | Quantities transfused | ||
| pRBC | 28 (82) | 4 (2-8) | 33 (97) | 5 (4-12) | 0.1352 |
| FFP28 (82) | 4 (2-7) | 30 (88) | 6.5 (4-14) | 0.0152 | |
| Platelets | 27 (79) | 2 (1-4) | 24 (71) | 2 (0-4) | 0.7040 |
| Cryoprecipitate | 25 (73) | 2 (0-3) | 19 (56) | 1 (0-2) | 0.0339 |
| Cell Saver | 19 (56) | 1 (0-3) | 25 (74) | 2 (0-6) | 0.1211 |
| Total Product Use | N/A | 14.5 (8-27) | N/A | 17 (10-39) | 0.1013 |
| pRBC + Cell Saver | N/AROT | 5.5 (2-11) EM | N/ACC | 8 (4-16) T | 0.0661 |
| Blood Loss in OR (mL) | 2000 | (1500-3375) | 3000 | (2000-7750) | 0.0375 |
All patients were included in the data set, regardless of transfusion status. The data is expressed as numbers and percentages or as medians and interquartile ranges. All products listed are in units, including combinations and totals. The number of patients transfused for each category was not statistically significant between the ROTEM/Conventional cohorts. P-values are for quantities transfused.
Median ICU length of stay, 30- and 60- day mortality were the same in both groups. Two patients died in each group; causes of death were liver failure due to primary graft non-function in the ROTEM and cardiac arrhythmia in the CCT group (Table 1).
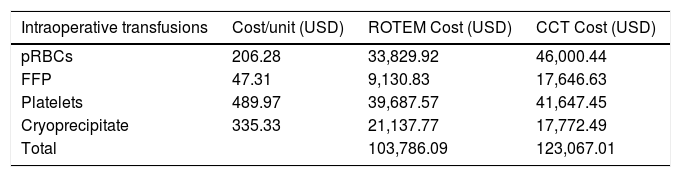
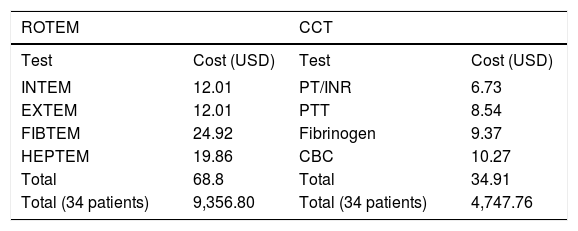
Direct cost results for blood products and testing are presented in tables 4 and 5. The total direct cost of blood products was less in the ROTEM group ($103,786.09 vs $123,067.01), table 4. Head to head cost of ROTEM laboratory testing was higher than CCT ($9,356.80 vs. $4,747.76), table 5. However, the overall cost of blood products plus laboratory testing was less in the ROTEM group ($113, 142.89 vs. $127,814.77).
Direct cost of blood products during OLT.
| Intraoperative transfusions | Cost/unit (USD) | ROTEM Cost (USD) | CCT Cost (USD) |
|---|---|---|---|
| pRBCs | 206.28 | 33,829.92 | 46,000.44 |
| FFP | 47.31 | 9,130.83 | 17,646.63 |
| Platelets | 489.97 | 39,687.57 | 41,647.45 |
| Cryoprecipitate | 335.33 | 21,137.77 | 17,772.49 |
| Total | 103,786.09 | 123,067.01 |
All patients were included in the data set, regardless of transfusion status. USD: United States Dollar.
Cost comparison of ROTEM and Conventional Coagulation Tests.
| ROTEM | CCT | ||
|---|---|---|---|
| Test | Cost (USD) | Test | Cost (USD) |
| INTEM | 12.01 | PT/INR | 6.73 |
| EXTEM | 12.01 | PTT | 8.54 |
| FIBTEM | 24.92 | Fibrinogen | 9.37 |
| HEPTEM | 19.86 | CBC | 10.27 |
| Total | 68.8 | Total | 34.91 |
| Total (34 patients) | 9,356.80 | Total (34 patients) | 4,747.76 |
Costs were obtained from OSUWMCs laboratory and from Tem systems, Inc. USD: United States Dollar.
In our comparison of ROTEM with conventional coagulation testing in liver transplant recipients, we found that the implementation of a ROTEM-based transfusion protocol during OLT led to significantly less intraoperative blood loss. We also noticed a decrease in transfusion of FFP and increase in cryoprecipitate, in the ROTEM group, as compared to the CCT group. Moreover, a decrease in total direct cost of blood products plus laboratory tests was observed in the ROTEM group.
Reduction in blood loss with the help of ROTEM is of clinical importance. Though intraoperative blood loss can be a somewhat subjective metric as it is estimated by the surgeon/anesthesiologist, the simultaneous decrease in salvaged blood use in the ROTEM group is supportive of these results. A Cochrane review by Gurusamy, et al.16 also showed reduced blood loss and blood transfusion requirements with the use of thromboelastography in two included trials. Other studies do show reduction in blood transfusion but none is able to demonstrate reduced blood loss.5-7,17
Reduction in FFP transfusion and increase in cryo-precipitate in the ROTEM group in our study is in contrast to the recent study by Roullet, et al.,6 which did not find any difference in these outcomes with implementation of their ROTEM-based transfusion algorithm. However, early study of ROTEM by Roullet, et al. resulted in an increased amount of fibrinogen transfusion.18 Based on the lessons learned from early protocol they changed their transfusion policy resulting in minimized FFP use in their recently published study.6 Authors of this study have also admitted to their change in practice evolved between 2008-2009 and 2012, and resulting low transfusion rate that was probably difficult to lower more, particularly for FFP.
Several previous studies have shown a decrease in transfusion of FFP with some reporting a decrease in overall blood product use with the implementation of a ROTEM-guided transfusion protocol.5-7,17 Our results build on these prior studies also demonstrated a significant decrease in FFP and increase in cryoprecipitate use. Prior to implementation of the ROTEM-based transfusion protocol at our institution, FFPs were preferentially given to correct coagulopathy. However, once the RO-TEM-based algorithm was applied, cryoprecipitate was instead transfused more due to ROTEM's better assessment of coagulation. In a large retrospective, multicenter study, Gorlinger, et al. found a decrease in transfusion of FFP, pRBCs, and platelet concentrate with an increase in fibrinogen concentrate and Prothrombin Complex Concentrate (PCC) in visceral surgery and OLT after implementation of a ROTEM-based transfusion protocol.7 Some blood products such as fibrinogen and PCC are not available for use in USA, therefore we used cryoprecipi-tate. Hence, our findings of increased use of cryoprecipi-tate need to be correlated with European centers’ increased fibrinogen use.
While many studies have looked at the effect of a RO-TEM-based transfusion algorithm on blood product use, few have compared the cost of blood products and coagulation factors before and after the implementation of such a protocol. Despite the increased cost of laboratory testing, use of the ROTEM-based transfusion algorithm at our study center decreased the direct costs by 11.5%. Similarly, Gorlinger, et al. found a savings of 36% when comparing direct costs of blood products and coagulation factor concentrates after implementation of a ROTEM-based transfusion protocol in visceral surgery and OLT in Germany.7 Similarly, Spalding, et al. found a savings of 44% after implementation of ROTEM-based transfusion protocol in cardiac surgery.11 Previously reported studies have not mentioned the cost of ROTEM testing, rather they reported the cost of blood products and coagulation factors. Despite the fact that the cost of ROTEM testing is almost twice the cost of conventional coagulation tests in our study, we felt these costs were important to elucidate. This finding suggests that it is not the cost of ROTEM testing but the reduction in cost of blood products linked to reduction in direct cost. Future studies are warranted to address total direct19,20 and indirect hospital costs between groups and we speculate there would be a greater cost savings in the ROTEM group.
A key distinction of this study is the subjects we included in our analysis. Previous studies have only involved patients receiving transfusion, with their amount of blood products transfused included in the analyses.6,7 Our study included all patients, regardless of transfusion status, as we felt this provided a more comprehensive assessment of in-traoperative transfusion requirements. Our study also included sicker patients with liver disease as reflected by a higher MELD score of 30 and 25 in ROTEM and CCT groups, respectively.
One of the major limitations of our study is that we compared the ROTEM group with a retrospective cohort. The ROTEM group may have had a better perioperative experience from the surgeons/anesthesiologists perspective and may not be absolutely comparable to the experience in the CCT group. This is an important bias in this analysis, however, the two groups of patients were consecutive and our team of surgeons, anesthesiologists and hepatologists was unchanged during that time period. The majority of studies have used a similar study design, and no randomized trial is available in this area to our knowledge.
The implementation of a ROTEM-based transfusion protocol reduced intraoperative blood loss and FFP use during OLT. Furthermore, the reduction in total direct costs of blood products plus coagulation tests after implementation of the ROTEM-based transfusion protocol readily supports its safety and utility during OLT. Further studies are needed in larger cohorts to confirm the efficacy of ROTEM in OLT and assess its potential use in patients with acute liver failure or cirrhosis requiring transfusions.
Abbreviations- •
aPTT: activated partial thromboplastin time.
- •
CCT: conventional coagulation tests.
- •
BMI: body mass index.
- •
FFP: fresh frozen plasma.
- •
ICU: intensive care unit.
- •
INR: international normalized ratio.
- •
LT: orthotopic liver transplantation.
- •
OSUWMC: The Ohio State University Wexner Medical Center.
- •
pRBCs: packed red blood cells.
- •
PT: prothrombin time.
- •
ROTEM: rotational thromboelastometry.
- •
SCT: Standard coagulation tests.
No grants or other financial support was received by any participating authors of this manuscript.
Conflict of InterestAll authors declare no conflicts of interest.
1AcknowledgementNone.
All authors participated in data collection, editing of the manuscript and contributed to the final version of this article.