Introduction. Radiofrequency ablation (RFA) is an effective and minimally invasive technique for the management of hepatic hemangiomas (HHs). This study aims to assess the safety and efficacy of laparoscopic RFA for HHs.
Material and methods. Forty-four patients with 50 hepatic hemangiomas (5-10 cm in diameter) undergoing laparoscopic RFA from January 2012 to May 2015 at three tertiary hospitals in China were retrospectively analyzed.
Results. Thirty-three patients with subcapsular hemangiomas were treated with a laparoscopic approach, and 11 patients with lesions in the liver parenchyma were treated with a combined laparoscopy and an ultrasound-guided percutaneous approach. No conversion to open surgery or two-step surgery occurred during the study period. Patients with small hemangiomas (< 7 cm) required a significantly shorter operating time (71.1 ± 20.18 min vs. 106 ± 23.55 min, p = 0.000) and fewer punctures compared with patients with large hemangiomas (> 7 cm) (4.61 ± 1.09 vs. 6.73 ± 1.01, P < 0.05). According to the Dindo-Clavien classification, 15 patients experienced 34 Grade 1 complications, and two had complications of Grade 3a. All complications were resolved by conservative treatment. Forty-three (86.0%) HHs in 38 patients were completely ablated after RFA, and 7 (14.0%) HHs in 6 patients were incompletely ablated. All patients were followed up for 6-24 months (mean 15 ± 6 months).
Conclusion. The data showed that laparoscopic RFA is an effective treatment for small (< 10 cm) HHs. While the incidence of postoperative complications remains high, the majority of complications are minor. Patients undergoing laparoscopic RFA for HHs, even for the small ones, should be carefully selected.
Hepatic hemangiomas (HHs) are common benign tumors occurring in human liver and consist of clusters of blood-filled cavities, lined by endothelial cells, fed by the hepatic artery, with an incidence rate of 0.7- 7% in the general population.1 With regard to sex distribution, it seems that women are more susceptible, as confirmed by relevant studies, with a reported 4.5:1 to 5:1 ratio of female to male cases.2,3 HHs are mainly single lesions, but multiple lesions are possible. The multiple lesions accounted for 2.3%, and even up to 20-30% of all cases as reported by some studies.1,4,5 In most circumstances, HHs do not show any signs and/or symptoms, and are often discovered incidentally during imaging examinations for unrelated conditions. If symptoms do occur, they are nonspecific, and common to many other diseases, especially digestive tract diseases. Pain in the right upper hemiabdomen is the most common complaint; others include decreased appetite, premature satiation sensation, nausea, vomiting, abdominal discomfort, sense of fullness, and early or late postprandial bloating. HHs can lead to complications depending on their size and location.6–8
Traditionally, surgical resection is the standard treatment for enlarged or symptomatic HHs.8 However, surgery requires a large abdominal incision and is associated with relatively long hospitalization and high risk of morbidity. In recent years, more and more surgeries have been performed in a minimally invasive manner either laparoscopically or with robotic approach.6 but can only be per formed by an experienced team. Other minimally invasive procedures such as selective or supra-selective angiographic embolization with polyvinyl alcohol or other substances by catheterization of the hepatic arteries, portal vein embolization and transhepatic ligation have also been attempted for the treatment of symptomatic HHs, but these techniques are not curative. Radiofrequency ablation (RFA) is an effective and minimally invasive technique for the management of HHs and has three main advantages. First, the benignity of the tumor does not require a safe margin of liver parenchyma surrounding the tumor. Second, as HHs are composed of blood-filled cavities, the effect of RFA can lead to the collapse of tumor tissue around the ablation zone. Third, due to the benignity of residual tumor after the initial treatment, residual tumor does not need to be treated urgently as it will not develop rapid tumor progression or metastasize to distant sites.9–11 However, one study has confirmed11 that RFA appears to be an inappropriate method for HHs due to the high rate of complications. The present study focused on the safety and efficacy of laparoscopic RFA for relatively small HHs using a retrospective multicenter design.
Material and MethodsPatientsFrom January 2012 to May 2015, patients undergoing laparoscopic RFA for HHs in the Foshan Hospital affiliated to Southern Medical University, Second Cancer Hospital of Heilongjiang Province and the Second Hospital affiliated to Harbin Medical University were analyzed retrospectively. The diagnosis of HH was based on at least two coincidental radiologic findings on ultrasound (US), contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Sixty-three patients with HHs refused traditional surgery and accepted RFA for the following indications:
- •
Hemangioma size was > 5 cm which induced abdominal pain or discomfort; and
- •
Hemangioma size was > 4 cm and increased by > 1 cm within 1 year of observation in regular follow-up imaging studies.
Forty-four patients with a hemangioma size of 5-10 cm were included in the study. For malignant liver tumors > 3 cm, RFA is not recommended in the guidelines, and unclear diagnosis of hemangioma is also a contraindication for RAF. Taking into account the thermal damage factors, if the hemangioma is close to the major blood vessels or bile ducts, this would be a relative contraindication for this technology. All patients gave written informed con sent before treatment. This study was approved by the Ethics Committees of each participating hospital and followed the guidelines of the Declaration of Helsinki. Preoperative patient and hemangioma characteristics, intraoperative details and postoperative outcomes including complications, treatment effectiveness and short-term outcomes were evaluated. Postoperative complications were classified by the modified Clavien-Dindo Classification system.12 Follow-up was defined as the time from laparoscopic RFA to the last clinical evaluation of the patient during the study period or death of the patient.
RFA proceduresThe RFA procedures in this study were performed using Cool-tip ACTC 2025 electrodes and an RF generator (Covidien Healthcare, Dublin, Ireland), and under general anesthesia via a standard laparoscopic approach. Intraoperative laparoscopic non-enhanced US was performed to locate hemangiomas in liver parenchyma. The electrode was advanced through normal liver parenchyma before reaching the target area to prevent hemangioma bleeding. RFA was then accomplished using a 17-gauge internally cooled-tip electrode with an exposed tip of 2 to 3 cm, delivering RF energy for 7-10 min for each intermediate step, and connected to an electrical generator (Covidien® TM, Dublin, Ireland). The abdominal wall puncture site was positioned as close to the target lesion as possible. The electrode was placed in the periphery of the lesion under US guidance. The RFA procedure was monitored by laparoscopic visualization and/or intraoperative US. Several insertions to treat overlapping zones were needed to complete the procedure. Tissue impedance was monitored throughout the procedure, and the maximum current was adjusted accordingly. A peristaltic pump was used to infuse 0 5°C normal saline solution into the lumen of the electrode at a rate to maintain a tip temperature < 20°C. The treatment process was guided by the appearance and progression of hyperechogeneity. After completing nodule ablation, the intrahepatic needle track was treated by thermocoagulation to avoid local bleeding. No laparoscopic biopsy of liver lesions was performed before ablation to avoid unnecessary bleeding. The procedure was performed without hepatic vascular inflow control.
All patients were followed up with contrast-enhanced CT or MRI for 1 month after ablation. Complete ablation was defined as no nodular or irregular enhancement adjacent to the ablated zone, as shown on enhanced CT or MRI scans. Incomplete ablation was defined as irregular, peripheral-enhanced foci in the ablated zone. In the case of complete ablation, subsequent CT or MRI examinations were repeated at 6-month intervals. In incomplete ablation, repeat RFA was not performed unless the residual tumor showed progression during the follow-up.
Statistical analysisIn patients with multiple lesions, the largest lesion was used for statistical analysis. Values were expressed as means ± standard deviation (SD). Continuous variables between groups were compared using the Student’s t test and analysis of variance. Differences in categorical data were analyzed using the χ2 test or Fisher’s exact test. Statistical analysis was performed using SPSS version 11.5.
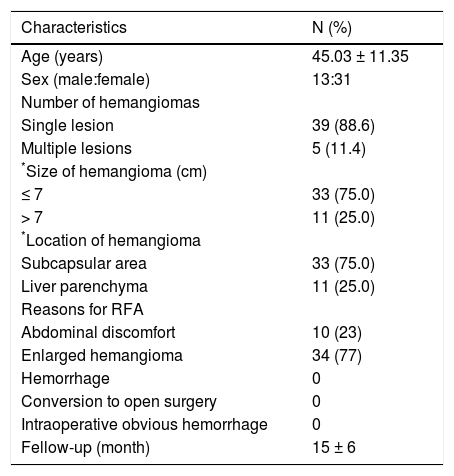
ResultsPatient characteristicsBetween January 2012 and May 2015, 44 patients with 50 HHs underwent laparoscopic ablation procedures. Patient and HH characteristics are shown in table 1. Most patients accepted RFA treatment due to enlarged hemangioma (34, 77%). Of these 44 patients, 39 patients (88.6%) had a single lesion, 4 (9.1%) patients had 2 lesions, and 1 (2.3%) patient had 3 lesions. And 38 (76.0%) lesions were located in the subcapsular area and 12(24.0%) lesions were located in the liver parenchyma. The patients with smaller hemangiomas (< 7 cm) required a significantly shorter operating time (71.1 ± 20.18 min vs. 106 ± 23.55 min, p = 0.000) and fewer punctures compared with patients with larger hemangiomas (> 7 cm) (4.61 ± 1.09 vs. 6.73 ± 1.01, p < 0.05) (Table 2).
Characteristics of hemangiomas.
| Characteristics | N (%) |
|---|---|
| Age (years) | 45.03 ± 11.35 |
| Sex (male:female) | 13:31 |
| Number of hemangiomas | |
| Single lesion | 39 (88.6) |
| Multiple lesions | 5 (11.4) |
| *Size of hemangioma (cm) | |
| ≤ 7 | 33 (75.0) |
| > 7 | 11 (25.0) |
| *Location of hemangioma | |
| Subcapsular area | 33 (75.0) |
| Liver parenchyma | 11 (25.0) |
| Reasons for RFA | |
| Abdominal discomfort | 10 (23) |
| Enlarged hemangioma | 34 (77) |
| Hemorrhage | 0 |
| Conversion to open surgery | 0 |
| Intraoperative obvious hemorrhage | 0 |
| Fellow-up (month) | 15 ± 6 |
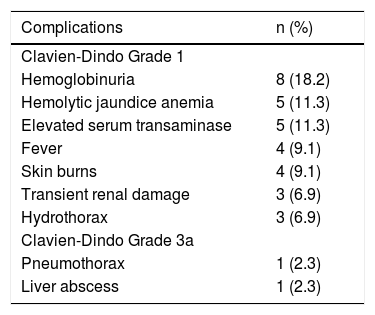
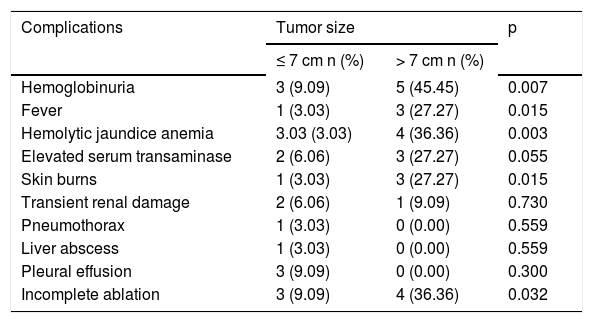
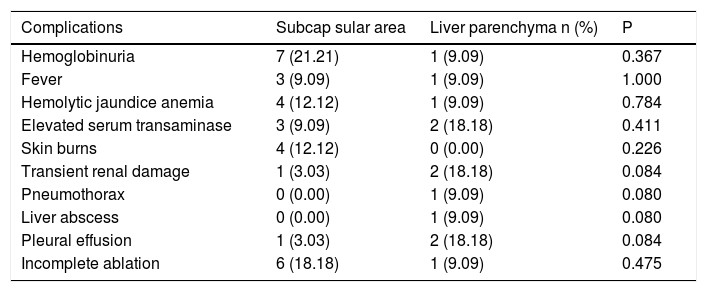
Thirty-six complications related to RFA were observed in 17 patients (Table 3). According to the Dindo-Clavien classification, 34 complications in 15 patients were grade 1. Hemoglobinuria was the most common postoperative complication and was found in 8 patients (18%). There was a trend for hemoglobinuria in patients with larger hemangiomas (9% vs. 45%, p = 0.007). Size of the hemangiomas was also associated with other postoperative complications, including fever, hemolytic anemia, elevated serum transaminase and skin burns (Table 4). However, lesions located in the subcapsular area or liver parenchyma were not involved in these postoperative complications (Table 5). Two major complications of Grade 3a (lower pneumothorax and liver abscess) were observed in 2 patients. All complications were resolved by conservative treatment.
Complications of laparoscopic RFA for hepatic hemangiomas in 44 patients.
| Complications | n (%) |
|---|---|
| Clavien-Dindo Grade 1 | |
| Hemoglobinuria | 8 (18.2) |
| Hemolytic jaundice anemia | 5 (11.3) |
| Elevated serum transaminase | 5 (11.3) |
| Fever | 4 (9.1) |
| Skin burns | 4 (9.1) |
| Transient renal damage | 3 (6.9) |
| Hydrothorax | 3 (6.9) |
| Clavien-Dindo Grade 3a | |
| Pneumothorax | 1 (2.3) |
| Liver abscess | 1 (2.3) |
Hemoglobinuria: examination of urinary sediments. Hemolytic jaundice anemia: total bilirubin > 34.2 mmol/L. Elevated serum transaminase: aspartate aminotransferase and alanine aminotransferase > 80 U/L. Fever: > 38 °C. Transient renal damage: creatinine > 133 mmol/L.
Complications of laparoscopic RFA in patients with different tumor size.
| Complications | Tumor size | p | |
|---|---|---|---|
| ≤ 7 cm n (%) | > 7 cm n (%) | ||
| Hemoglobinuria | 3 (9.09) | 5 (45.45) | 0.007 |
| Fever | 1 (3.03) | 3 (27.27) | 0.015 |
| Hemolytic jaundice anemia | 3.03 (3.03) | 4 (36.36) | 0.003 |
| Elevated serum transaminase | 2 (6.06) | 3 (27.27) | 0.055 |
| Skin burns | 1 (3.03) | 3 (27.27) | 0.015 |
| Transient renal damage | 2 (6.06) | 1 (9.09) | 0.730 |
| Pneumothorax | 1 (3.03) | 0 (0.00) | 0.559 |
| Liver abscess | 1 (3.03) | 0 (0.00) | 0.559 |
| Pleural effusion | 3 (9.09) | 0 (0.00) | 0.300 |
| Incomplete ablation | 3 (9.09) | 4 (36.36) | 0.032 |
Complications of laparoscopic RFA in patients with different tumor location.
| Complications | Subcap sular area | Liver parenchyma n (%) | P |
|---|---|---|---|
| Hemoglobinuria | 7 (21.21) | 1 (9.09) | 0.367 |
| Fever | 3 (9.09) | 1 (9.09) | 1.000 |
| Hemolytic jaundice anemia | 4 (12.12) | 1 (9.09) | 0.784 |
| Elevated serum transaminase | 3 (9.09) | 2 (18.18) | 0.411 |
| Skin burns | 4 (12.12) | 0 (0.00) | 0.226 |
| Transient renal damage | 1 (3.03) | 2 (18.18) | 0.084 |
| Pneumothorax | 0 (0.00) | 1 (9.09) | 0.080 |
| Liver abscess | 0 (0.00) | 1 (9.09) | 0.080 |
| Pleural effusion | 1 (3.03) | 2 (18.18) | 0.084 |
| Incomplete ablation | 6 (18.18) | 1 (9.09) | 0.475 |
No conversion to open surgery or two-step surgery occurred during the study period. Forty-three hemangiomas in 38 patients were completely ablated after RFA and 7 hemangiomas in 6 patients were incompletely ablated, and an obvious > 1 cm enhancement on the peripheral rim of the ablated tumors was seen on follow-up CT or MRI. Patients were followed for 6-24 months (mean, 15 ± 6 months). In patients with incomplete ablation, abdominal discomfort due to hemangiomas was resolved.
DiscussionLiver hemangiomas were initially treated with surgery. In 1989, hemangiomas were first treated with hepatic resection by Hermann P fannestil.13 Hepatic resection or enucleation is the most common therapy for this tumor.14 Recently, other therapies such as RFA, radiotherapy, transarterial embolization, chemotherapy, and liver transplantation, have been performed, but have not been proven uniformly effective. Interferon alpha 2a was also used for this disease, but it requires a long period of treatment with an unsatisfactory remission rate.6,15 RFA for HHs has three advantages. First, RFA for HHs does not require an ablative margin of normal hepatic parenchyma surrounding the tumor. Therefore, the target of ablation for HHs is clear, unlike that for malignant neoplasms. Second, wellperformed ablation of HHs can lead to the collapse of tumor tissue around the ablation zone, making RFA for HHs more efficient and easier than that for malignant hepatic lesions. Third, residual tumor, if present, does not develop rapid tumor progression or metastasize to distant sites, which is often seen in the follow-up of hepatic malignancies treated with RFA.9–11
Laparoscopic RFA not only offers a direct vision of the entire procedure, but also isolates the tumor from the abdominal viscera, and thus reduces the thermal injury to the adjacent tissues. In special situations, simultaneous procedures can be performed, such as cholecystectomy and laparoscopic liver resection. Furthermore, capnopneumoperitoneum has a beneficial physiopathological effect on RFA as it decreases the portal inflow by 40% allowing better thermal conduction, which contributes to greater ablation efficacy.13 In our experience, this treatment is very easy to perform and no conversion to open surgery occurred in our study. Although 38 / 44 patients were completely ablated after RFA, 6 / 44 patients were incompletely ablated, and an obvious > 1 cm enhancement on the peripheral rim of the ablated tumors was observed on follow-up CT or MRI. The tumor size is significantly reduced after RFA due to the adequate blood supply in a HH. Although residual phenomena are observed in some cases, the subjective symptoms of HHs are completely resolved. The generous blood supply in HHs can also induce hemolysis after RFA. Hemolysis can lead to various degrees of hemoglobinuria, hemolytic anemia, or even renal damage, depending on its severity. With larger HHs, the risk of complications is greater.11 In our study, we classified the HHs into two groups according to tumor size, hemoglobinuria, fever and hemolytic anemia were more likely to develop after RFA in patients with large HHs. These results were also found in HHs < 10 cm and 17 of 44 cases had complications. This was different from that seen in two previous studies. When considering HHs < 10 cm, we believe that the presence of complications may be due to the use of different standards and surgical strategies. Unlike previous clinical studies, hemangiomas were located in liver parenchyma in our study on US, and we suspect that this is one of the reasons for the different rates of complications compared to previous reports. We separated hemangiomas into those located in liver parenchyma and those located in the subcapsular area, and found no difference in the rate of complications between the two groups. A lack of sufficient experience in the initial stage of treating large HHs with RFA may also play a role in the occurrence of major complications. Several studies have reported a morbidity rate of 10 - 27% after resection or enucleation of HHs.16–19 Some studies focusing on RFA and open surgery showed that morbidity and mortality were similar in both groups.16
At present, most researches focus on subcapsular hemangiomas. Laparoscopic RFA has been shown to be effective and reasonably safe for the treatment of subcapsular hemangiomas 5 - 10 cm in size by two independent study groups.16,20 Our study is the first multicenter trial and includes tumors in the liver parenchyma. In contrast to previous studies, we used laparoscopic US to locate parenchymal lesions, and for those with liver surface lesions, intraoperative ultrasonography was performed routinely in the laparoscopic approach to enhance the capacity to determine real-time RF electrode placement and evaluate the efficacy of ablation.
There are limitations to our study, which include its retrospective nature, the lack of a control group, the short follow-up period and the relatively small number of patients evaluated. Traditional surgical approach was also selected in some patients with large hemangiomas. The feasibility of RFA is largely dependent on the operator s skills and experience, and the center s equipment adequacy. Our patients were managed based mainly on the surgeon s perspectives, making the results less applicable in nonsurgical settings. Further long-term outcomes and prospective randomized controlled trials are needed to define the role of laparoscopic RFA in the treatment of HHs, especially comparing to the open surgical resection.
In conclusion, laparoscopic RFA is an effective procedure for small HHs. The incidence of postoperative complications is relatively high, although the majority of them are minor, laparoscopic RFA, even for small HHs, still requires careful patient selection.
AcknowledgementsThis study was funded by a grant from Foshan Hospital affiliated to Southern Medical University, Guangdong Province, China.
Compliance With Ethical StandardsThis study was approved by the Ethics Committees of each participating hospital in this multi-center study, and followed the guidelines of the Declaration of Helsinki.
Competing InterestsThe authors declare that they have no competing interests.