Sarcopenia is a complication and independent risk factor for mortality in patients with liver cirrhosis.
AimTo assess the prevalence and influence of sarcopenia on overall survival in a cohort of cirrhotic patients with hepatocellular carcinoma managed in a tertiary center.
Material and MethodsAbdominal computed tomography of 92 consecutive hepatocellular carcinoma cirrhotic patients, enrolled and followed from 2004 to 2014, were retrospectively studied with a software analyzing the cross-sectional areas of muscles at third lumbar vertebra level. Data was normalized for height, skeletal muscle index (SMI) calculated and presence of Sarcopenia measured. Sarcopenia was defined by SMI ≤ 41 cm2/m2 for women and ≤ 53 cm2/m2 for men with body mass index (BMI) ≥ 25, and ≤ 43 cm2/m2 for men and women with BMI < 25, respectively.
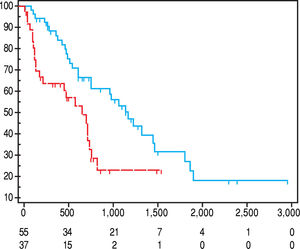
ResultsMedian age at diagnosis was 71.9 years (30.7-86.4) and BMI 24.7 (17.5-36.7), comparable in women 23.1, (17.5-36.7) and men 24.7 (18.4-36.7). A class of CHILD score and BCLC A prevailed (55.4% and 41.3%, respectively); metastatic disease was found in 12% of cases. Sarcopenia was present in 40.2% of cases, mostly in females (62.9%; p = 0.005). Mean overall survival was reduced in sarcopenic patients, 66 (95% CI 47 to 84) vs. 123 (95% CI 98 to 150) weeks (p = 0.001). At multivariate analysis, sarcopenia was a predictor of reduced overall survival, independent of age (p = 0.0027).
ConclusionsThis retrospective study shows high prevalence of sarcopenia among cirrhotic patients with hepatocellular carcinoma. Presence of sarcopenia was identified as independent predictor of reduced overall survival. As easily measurable by CT, sarcopenia should be determined for prognostic purposes in this patient population.
Hepatocellular carcinoma (HCC) is the 7th most common tumor among males (4% of all cancers), the 13th in females (2.3% of all cancers), and the 5th and 7th leading cause of malignancy-related mortality among men and woman, respectively (4.5% of all malignancy-related mortality).1 It represents more than 90% of primary liver cancers and is a major global health problem.2,3 Most cases of HCC in the Western world occur in the setting of cirrhosis, therefore prognosis is not only influenced by tumor-related factors, but also by those due to cirrhosis. Sarcopenia (Src), or muscle wasting, is defined as the physiological loss of lean body mass (BM) due to the loss of muscle mass (MM), and of its functional capacity,4 independently from fat mass loss. All human beings begin to lose MM and function since the age of 40 years. This physiological loss has been estimated to be around 8% per decade up to age 70, increasing later to 15% per decade (primary Src).5-6 Reduction of protein synthesis is the leading cause of BM loss. Secondary Src is instead said to be present when a disease processes, such as a neo-plasia, determines a prevailing proteic catabolism.7 Src has been observed in approximately 40% of patients with liver cirrhosis,8-10 significantly affects quality of life,10 increases the risk for development of other cirrhosis complications such as encephalopathy,11 post-transplantation mortality,12 infections, and effectiveness of intra-arterial treatments.13 It also affects mortality caused by neoplastic diseases, as observed in cirrhotic patients selected to undergo HCC treat-ment9 too.
However, in spite of the large number of articles in the literature, there is currently neither agreement nor uniformity of choice on the diagnostic criteria to define the presence of Src, nor on the techniques to assess it.5
The aim of our study was to determine the prevalence of Src in a cohort of cirrhotic patients at the first diagnosis of HCC treated in a tertiary clinical center, quantitating it using readily available imaging based techniques, and to evaluate its influence on patients survival.
Material and MethodsWe conducted a retrospective study on 121 consecutive cirrhotic patients at their first diagnosis of HCC attending our outpatient clinic enrolled from June 2004 to August 2014. Available imaging studies were evaluated and considered assessable for the study if obtained by CT scanning technique and if the transverse images allowed evaluation of the muscular structures at the level of third lumbar vertebra (L3). Skeletal muscle cross-sectional area was evaluated as follows: a transverse CT image, with multiplanar reconstruction from L3, was selected from the radiologic study of each patient. Images were analyzed by the dedicated software Slice Omatic V 5.0 (Montreal, Quebec, Canada Tomovision), marking the muscles areas by Hounsfield unit thresholds of -29 to +150, and then summing up the total cross sectional area at that level. The muscle bundles present in that area are the psoas, paraspinal, abdominal transverse rectus, internal and the external obliques. The cross-sectional areas obtained are then normalized for patient height, obtaining the skeletal muscle index (SMI), expressed as cross-sectional muscle area/height2. Src was defined to be present when SMI was ≤ 41 cm2/m2 in women, and ≤ 53 cm2/m2 for men with a body mass index (BMI) ≥ 25, and ≤ 43 cm2/m2 for men and women with BMI < 25, as derived from the published results of a CT-based study of 1,475 patients with solid tumors in which these cutoffs were identified by optimal stratification.14
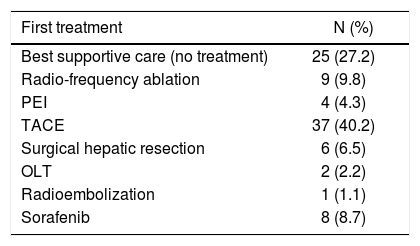
At baseline, each patient underwent complete medical and pharmacological history; causes of cirrhosis were ascertained, and physical examination performed (to establish the presence of ascites and/or hepatic encephalopathy); anthropometric parameters (sex, weight, height, BMI), and relevant liver biochemistries were obtained and entered in a dedicated database. At first diagnosis of HCC patients were allocated to management according to the BCLC classification, i.e.: Ablation [radio-frequency ablation (RF) or percutaneous ethanol injection (PEI)]; trans-arterial chemoembolization (TACE); surgical hepatic resection; orthotopic liver transplant (OLT); sorafenib; best supportive care (no treatment). One patient was treated with radioembolization, a strategy currently cited in a recent review of the BCLC classification as a treatment in development.15 Presence of comor-bidities, defined as the presence of additional medical conditions co-occurring with a primary disease, such as arterial hypertension, type II diabetes, chronic renal failure, obstructive chronic bronchitis (OCB), cardiovascular and neurological diseases and other residual categories, as well the presence of previous neoplasm, were also recorded.
Statistical analysisComparison between groups was performed by Fisher’s exact test for discrete variables and by the Mann-Whitney test for continuous, non-parametric unpaired variables. Survival curves were compared by Kaplan-Meier analysis. Limit of statistical significance was set at p < 0.05. The results are expressed as median (range) for continuous variables and as number/total (%) for continuous, non-parametric unpaired discrete variables, unless otherwise specified. Overall survival is reported as mean (95% confidence interval). Statistical analysis were performed using MedCalc for Window version 12.7.
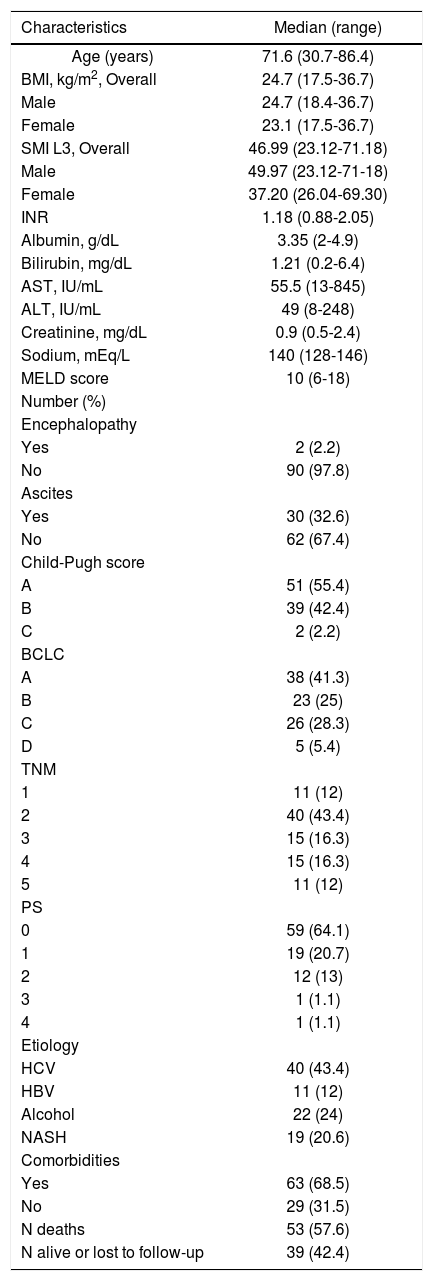
ResultsComplete data was available in 92 HCC pts [27 females (29.4%), 65 males (70,6%)]. The clinical and biochemical characteristics of the study patients are shown in table 1 and patients management strategy allocation at first HCC diagnosis are listed in table 2. The comparison of clinical e biochemical characteristics between sarcopenic and non sarcopenic patients are shown in table 3. Median age at diagnosis was 71.6 years (30.7-86.4), and median BMI was 24.7 (17.5-36.7), similar among women and men. Viral infection was the cause of liver disease in around 55% of patients, while alcohol and non-alcoholic steato-hepatitis contributed almost equally for the remaining HCC cases. Comorbidities are listed in table 4.Distribution by Child score showed a prevalence of A class (51/92, 55.4%), while median value of model for end-stage liver disease (MELD) score was 10 (6-18). Metastatic disease was present in 11/92 (12%). The distribution by BCLC showed a prevalence of A class (38/92, 41.3%). No significant difference in management strategy allocation at first HCC diagnosis was observed among non sarcopenic and sarcopenic patients (data not shown).
Clinical and biochemical characteristics of 92 HCC patients at diagnosis. Data are expressed as Median (range) or number (%).
| Characteristics | Median (range) |
|---|---|
| Age (years) | 71.6 (30.7-86.4) |
| BMI, kg/m2, Overall | 24.7 (17.5-36.7) |
| Male | 24.7 (18.4-36.7) |
| Female | 23.1 (17.5-36.7) |
| SMI L3, Overall | 46.99 (23.12-71.18) |
| Male | 49.97 (23.12-71-18) |
| Female | 37.20 (26.04-69.30) |
| INR | 1.18 (0.88-2.05) |
| Albumin, g/dL | 3.35 (2-4.9) |
| Bilirubin, mg/dL | 1.21 (0.2-6.4) |
| AST, IU/mL | 55.5 (13-845) |
| ALT, IU/mL | 49 (8-248) |
| Creatinine, mg/dL | 0.9 (0.5-2.4) |
| Sodium, mEq/L | 140 (128-146) |
| MELD score | 10 (6-18) |
| Number (%) | |
| Encephalopathy | |
| Yes | 2 (2.2) |
| No | 90 (97.8) |
| Ascites | |
| Yes | 30 (32.6) |
| No | 62 (67.4) |
| Child-Pugh score | |
| A | 51 (55.4) |
| B | 39 (42.4) |
| C | 2 (2.2) |
| BCLC | |
| A | 38 (41.3) |
| B | 23 (25) |
| C | 26 (28.3) |
| D | 5 (5.4) |
| TNM | |
| 1 | 11 (12) |
| 2 | 40 (43.4) |
| 3 | 15 (16.3) |
| 4 | 15 (16.3) |
| 5 | 11 (12) |
| PS | |
| 0 | 59 (64.1) |
| 1 | 19 (20.7) |
| 2 | 12 (13) |
| 3 | 1 (1.1) |
| 4 | 1 (1.1) |
| Etiology | |
| HCV | 40 (43.4) |
| HBV | 11 (12) |
| Alcohol | 22 (24) |
| NASH | 19 (20.6) |
| Comorbidities | |
| Yes | 63 (68.5) |
| No | 29 (31.5) |
| N deaths | 53 (57.6) |
| N alive or lost to follow-up | 39 (42.4) |
Patient management at first HCC diagnosis.
| First treatment | N (%) |
|---|---|
| Best supportive care (no treatment) | 25 (27.2) |
| Radio-frequency ablation | 9 (9.8) |
| PEI | 4 (4.3) |
| TACE | 37 (40.2) |
| Surgical hepatic resection | 6 (6.5) |
| OLT | 2 (2.2) |
| Radioembolization | 1 (1.1) |
| Sorafenib | 8 (8.7) |
Comparison of clinical and biochemical characteristics among sarcopenic and non sarcopenic patients.
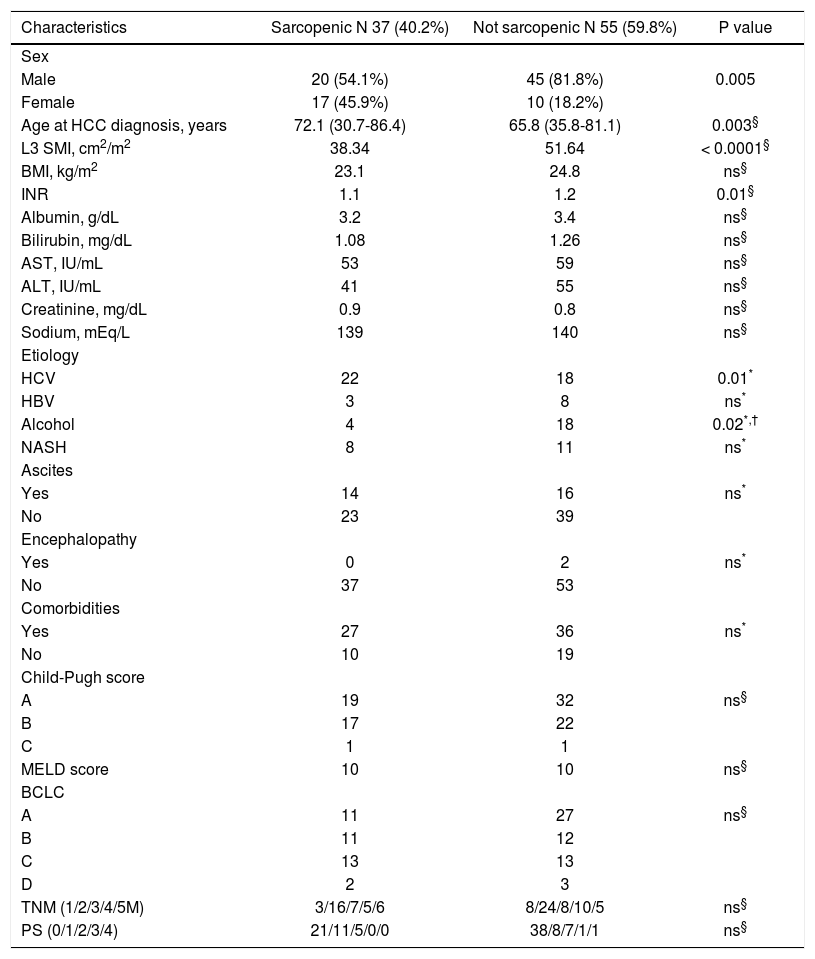
| Characteristics | Sarcopenic N 37 (40.2%) | Not sarcopenic N 55 (59.8%) | P value |
|---|---|---|---|
| Sex | |||
| Male | 20 (54.1%) | 45 (81.8%) | 0.005 |
| Female | 17 (45.9%) | 10 (18.2%) | |
| Age at HCC diagnosis, years | 72.1 (30.7-86.4) | 65.8 (35.8-81.1) | 0.003§ |
| L3 SMI, cm2/m2 | 38.34 | 51.64 | < 0.0001§ |
| BMI, kg/m2 | 23.1 | 24.8 | ns§ |
| INR | 1.1 | 1.2 | 0.01§ |
| Albumin, g/dL | 3.2 | 3.4 | ns§ |
| Bilirubin, mg/dL | 1.08 | 1.26 | ns§ |
| AST, IU/mL | 53 | 59 | ns§ |
| ALT, IU/mL | 41 | 55 | ns§ |
| Creatinine, mg/dL | 0.9 | 0.8 | ns§ |
| Sodium, mEq/L | 139 | 140 | ns§ |
| Etiology | |||
| HCV | 22 | 18 | 0.01* |
| HBV | 3 | 8 | ns* |
| Alcohol | 4 | 18 | 0.02*,† |
| NASH | 8 | 11 | ns* |
| Ascites | |||
| Yes | 14 | 16 | ns* |
| No | 23 | 39 | |
| Encephalopathy | |||
| Yes | 0 | 2 | ns* |
| No | 37 | 53 | |
| Comorbidities | |||
| Yes | 27 | 36 | ns* |
| No | 10 | 19 | |
| Child-Pugh score | |||
| A | 19 | 32 | ns§ |
| B | 17 | 22 | |
| C | 1 | 1 | |
| MELD score | 10 | 10 | ns§ |
| BCLC | |||
| A | 11 | 27 | ns§ |
| B | 11 | 12 | |
| C | 13 | 13 | |
| D | 2 | 3 | |
| TNM (1/2/3/4/5M) | 3/16/7/5/6 | 8/24/8/10/5 | ns§ |
| PS (0/1/2/3/4) | 21/11/5/0/0 | 38/8/7/1/1 | ns§ |
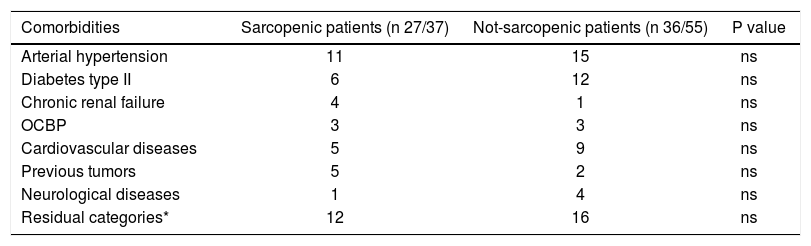
Distribution of comorbidities.
| Comorbidities | Sarcopenic patients (n 27/37) | Not-sarcopenic patients (n 36/55) | P value |
|---|---|---|---|
| Arterial hypertension | 11 | 15 | ns |
| Diabetes type II | 6 | 12 | ns |
| Chronic renal failure | 4 | 1 | ns |
| OCBP | 3 | 3 | ns |
| Cardiovascular diseases | 5 | 9 | ns |
| Previous tumors | 5 | 2 | ns |
| Neurological diseases | 1 | 4 | ns |
| Residual categories* | 12 | 16 | ns |
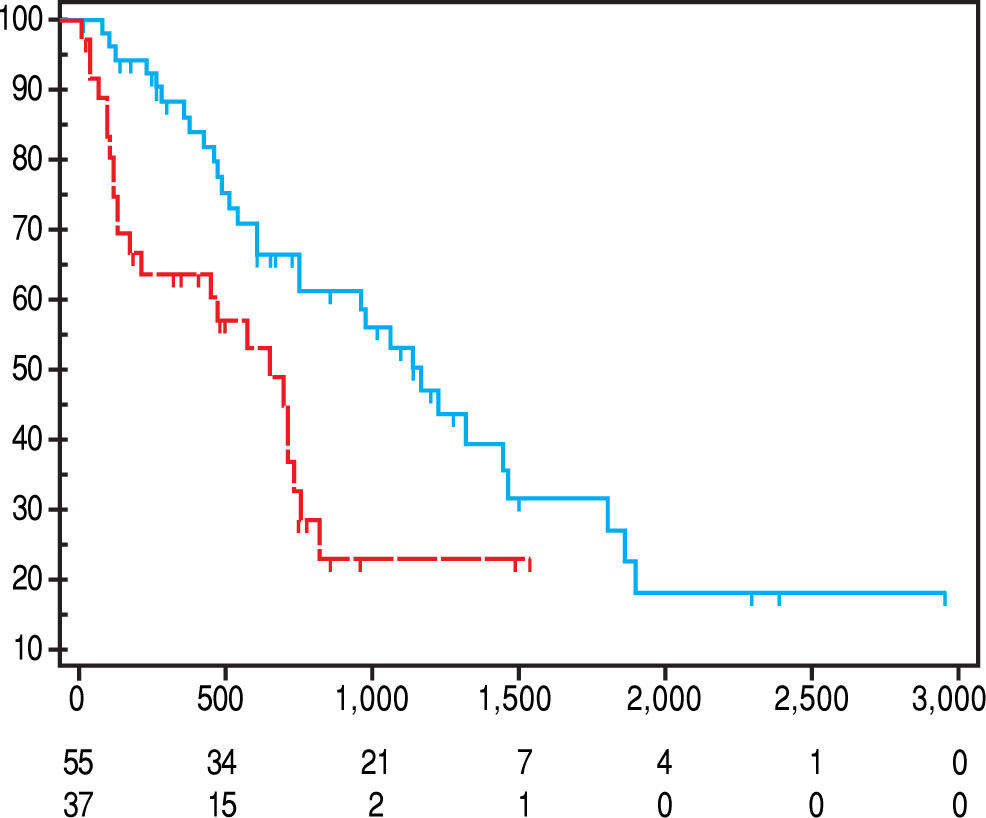
Overall, median value of SMI L3 was 47 cm2/m2 (23.1-71.2) with 50 cm2/m2 (23.1-71.2) in males and 37.2 (26-69.3) in females (p = 0.005). Src was present in 37/92 (40.2%) patients. As shown in table 3, Src was significantly more prevalent in females, and sarcopenic patients were older at HCC diagnosis. The prevalence of Src was significantly higher among HCV-related HCC patients as compared to those due to alcohol. Prevalence of Src was not different among different TNM, Child-Pugh, MELD and BCLC groups (Table 3). Mean Overall Survival was 123 weeks (95% CI: 98-150) in non-sarcopenic, while it was shorter in sarcopenic (66 weeks; 95% CI: 47-84, log-rank, p = 0.005, Figure 1).
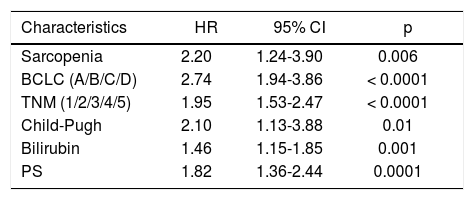
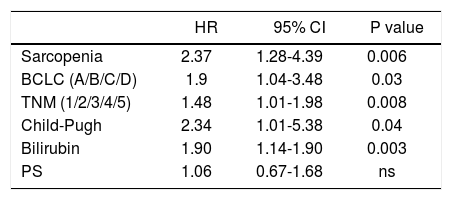
Univariate Cox regression analysis showed that the presence of Src, bilirubin level, performance status, presence of liver decompensation (Child-Pugh ≤ B7 vs ≥ B8), TNM staging, and BCLC class were also significantly associated with survival (Table 5). At multivariate analysis, all these parameters, with the exception of the performance status, were confirmed to be independently associated with an increased mortality risk (p < 0.0001; Table 6). Comorbidities were equally distributed among the 2 groups (73% vs. 65.4%, p = NS; Table 4), and when groups were analyzed for their presence, Src remained an independent risk factor of mortality.
Univariate analysis of possible risk factors for overall survival of hepatocellular carcinoma patients by the Cox propor- tional hazards model.
| Characteristics | HR | 95% CI | p |
|---|---|---|---|
| Sarcopenia | 2.20 | 1.24-3.90 | 0.006 |
| BCLC (A/B/C/D) | 2.74 | 1.94-3.86 | < 0.0001 |
| TNM (1/2/3/4/5) | 1.95 | 1.53-2.47 | < 0.0001 |
| Child-Pugh | 2.10 | 1.13-3.88 | 0.01 |
| Bilirubin | 1.46 | 1.15-1.85 | 0.001 |
| PS | 1.82 | 1.36-2.44 | 0.0001 |
Multivariate analysis of possible risk factors for overall survival of hepatocellular carcinoma patients by the Cox propor- tional hazards model.
| HR | 95% CI | P value | |
|---|---|---|---|
| Sarcopenia | 2.37 | 1.28-4.39 | 0.006 |
| BCLC (A/B/C/D) | 1.9 | 1.04-3.48 | 0.03 |
| TNM (1/2/3/4/5) | 1.48 | 1.01-1.98 | 0.008 |
| Child-Pugh | 2.34 | 1.01-5.38 | 0.04 |
| Bilirubin | 1.90 | 1.14-1.90 | 0.003 |
| PS | 1.06 | 0.67-1.68 | ns |
In this retrospective, single-center study, Src assessed by a measurable methodology, was present in 40.2% of HCC patients followed at our institution. This percentage is only marginally superior to that observed by Meza-Jun-co (30%)16 in patients assessed for liver transplant for HCC, coherent with those reported in cirrhotic patients with HCC, and observed in patients with other solid tu-mors.7,9,17 However, and differently from other series, our study population consisted of cirrhotic patients at their first diagnosis of HCC, and is thus more representative of what encountered in daily practice. In this unselected population, we observed that Src is a strong and independent predictor of HCC-related mortality in cirrhotic patients together with increased bilirubin, presence of de-compensated cirrhosis, performance status, TNM staging, and BCLC class. Interestingly, prevalence of Src was not different throughout the different TNM, Child-Pugh, MELD, and BCLC groups, as also reported by Tandon Puneeta study.9 A recent study on a rather large Japanese population18 also identified the presence of Src as a prognostic marker of decreased survival. However, differently from our population, more than 37% of patients enrolled in this study had already undergone HCC treatment, thus affecting the interpretation of the net effect of Src on sur-vival.18 However, the ethnic background, the cutoff used to define the presence of Src and the median aged of patients evaluated in this study differed from those of our population. Also, the causes of liver disease were not available for evaluation.
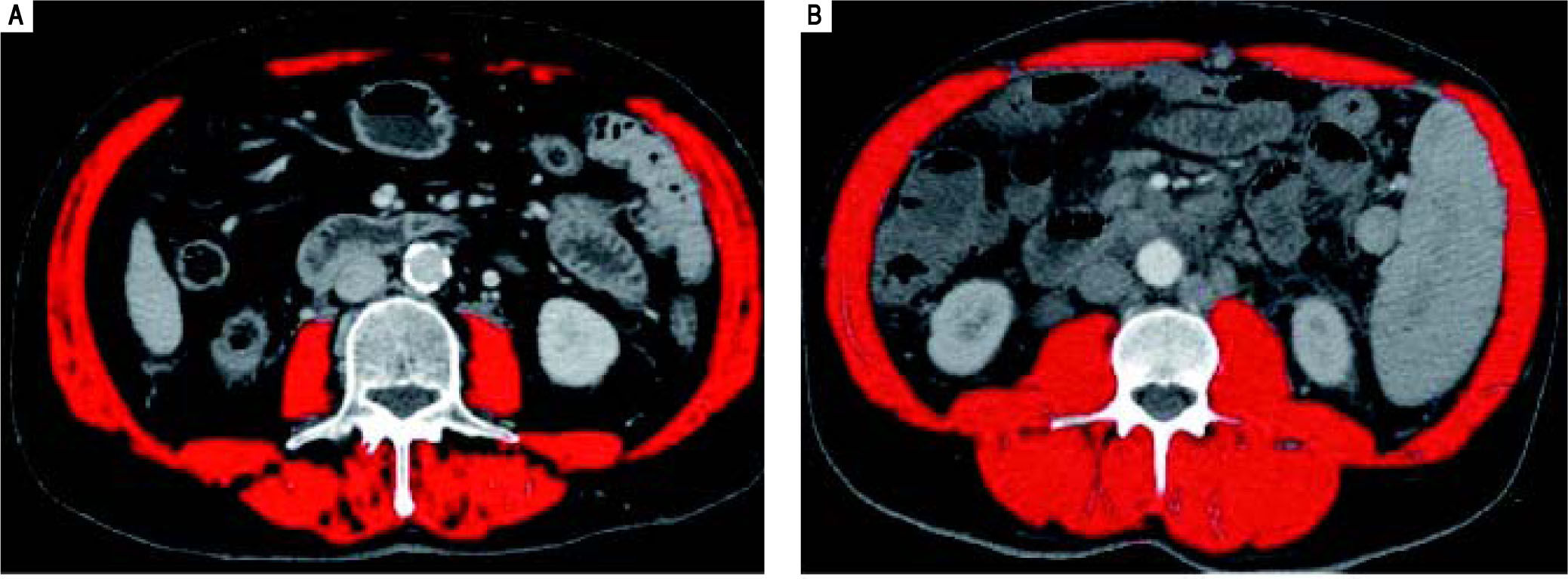
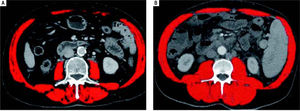
Systematic reviews confirm that any staging system with prognostic implications for HCC patients should take into account the tumor stage, the stage of liver failure, and the presence of disease-related symptoms (i.e. performance status).19 Several staging systems for HCC have been developed.20-22 The Okuda system, which includes measures of the tumor, ascites, bilirubin and albumin, is applicable only in advanced stages, but it is more limited if evaluated at earlier stages. The Gretch score was based on a study in 761 patients of whom more than half had un-treatable HCC.20 The CLIP score (Cancer of the Liver Italian Program) considers three tumor-related variables (alpha-fetoprotein concentration, size, and presence/absence of portal vein thrombosis), plus the Child-Pugh score. However, validation studies detected inconsistencies in survival curves, and it does not provide different therapeutic options for different disease stages.21 Currently, the staging classification proposed by the Barcelona Clinic Liver Cancer (BCLC)22 is the most widespread, and it is adopted by major international guidelines.2,23-25 It considers most of the previously listed parameters, it associates them to therapeutic indications in a multidimensional model of staging and management, and it has been pro-spectively validated in different clinical settings.3,22,26 Nevertheless, it should not be considered a prognostic model.27 These models and staging systems, however do have limitations in providing a comprehensive tool able to fully consider all the complex interactions between the underlying liver cirrhosis and HCC. A recent comprehensive review of prognostic scores for HCC, analyzing 72 studies with a total of 23,968 patients, concluded that in compensated cirrhosis, tumor-related factors are the most influential on survival, while in decompensated cirrhosis, factors expressing deterioration of hepatic function become more relevant in predicting survival.28 Thus, evaluation of Src might contribute to further fine tune the prognostic power of these models and 12 staging systems. We also observed that BMI does not correlate with Src, thus stressing the concept that the latter condition should not be given for granted in underweight patients, and that it can also might go unnoticed in HCC cirrhotic patients of any BMI (as shown in Figure 2). In fact, even if no difference in Src prevalence was detected in NASH (nonalcoholic steatohepatitis) patients as compared to other groups, we observed that this condition was present in 50% of overweight patients (BMI > 25 < 30) and in 15% of grade I obese patients (BMI ≥ 30) from our series, confirming the existence of the condition called sarco-penic obesity.29-31 Thus the use of CT-based techniques in these cirrhotic patients with HCC will allow for the detection and measurement of this independent prognostic index. This method also overcomes the limits intrinsic to bioimpedance and anthropometric measurement, hampered by elevated BMI and ascites.18,29-31 Other techniques to measure MM have been proposed,32 but all have limitations, influencing their diffusion in clinical practice. Some, as those used to test muscle strength, such as handgrip strength by dynamometer and flexion/ extension of the knee, requiring equipment and personnel training, are difficult to apply in clinical practice. Some other estimate body fat and lean BM (bioimped-ance and anthropometric measurements of arm circumference, calf and skinfold), but do not consider or correct for potential confounding factors, such as age-dependent, differential fat deposition, thus also affecting their relia-bility.4 Also DEXA (Dual Energy X-ray Absorptiometry) measurement has been used to evaluate MM.33 However, radiological imaging such as computed tomography (CT), and magnetic resonance imaging are currently credited as the most reliable techniques for the quantification of MM.17 Using these radiological imaging techniques, the diagnosis and quantification of Src has been obtained by measuring MM, defined as the cross sectional area of the muscular structures present at the level of a section passing through the third lumbar vertebra (L3).4,17 This parameter is measurable, replicable and reproducible, and this technique overcomes the technical difficulties due to the presence of ascites.14 Furthermore, automated software programs are available to facilitate the measurement and easy to find and relatively affordable.
Thus CT-based techniques are able to objectively and reproducibly measure Src and, considering that imaging evaluation is always performed at baseline for staging purposes, Src could be derived by these studies at marginally increased costs, acquiring a further independent prognostic index to plan appropriate patients management.
In conclusion, we believe that modern medical oncology should not be confined to the study of cancer treatments, and that it should also address clinical research toward supportive care, including nutrition assessment and management. Src assessment should be integrated in the evaluation of all cirrhotic patients with HCC at baseline diagnosis, since it is an independent predictor factor of mortality, and its availability will be of help in optimizing accurate prognosis and treatment decisions. Prospective studies are however needed. Additionally, it has to be ascertained in interventional studies if treatment of Src might improve the survival of patients.
Our data suggests that Src is not only frequent in cir-rhotic HCC patients at first diagnosis but it is also a predictor of patients survival. Its evaluation should be included in the baseline workup of these patients to integrate our prognostic scores and provide a better tailored management strategy.
Abbreviations- •
BCLC: Barcelona Clinic Liver Cancer.
- •
BMI: body mass index.
- •
CI: confidence interval.
- •
CLIP: Cancer of the Liver Italian Program.
- •
CT: computerized tomography.
- •
DEXA: dual energy X-ray absorptiometry.
- •
HCC: hepatocellular carcinoma.
- •
L3: lumbar vertebra 3.
- •
MELD: Model for End-stage Liver Disease.
- •
MM: muscle mass.
- •
NASH: non-alcoholic steatohepatitis.
- •
NS: not significant.
- •
SMI: skeletal muscle index.
- •
Src: sarcopenia.
- •
TNM: tumor nodes metastases.
The authors have no disclosures to report.
Financial SupportNo financial support.
AcknowledgmentsA special thank you to doctor Ada D’Aniello for his thorough review language.
Conflict of InterestThe authors have no disclosures to report.