Solid organ transplant (SOT) recipients remain vulnerable to developing more severe COVID-19 courses due to immunosuppression and the high prevalence of comorbidities. Hence, adequate protection against COVID-19 is crucial. Early after the COVID-19 vaccine implementation, it became evident that immunocompromised hosts have a reduced humoral and cellular response after a complete SARS-CoV-2 vaccine regimen, this was consistent irrespective of the vaccine platform used [1]. Low seroconversion rates have been reported in SOT recipients after the second dose of SARS-CoV-2 vaccines [2]. Consequently, health authorities have recommended administering a third dose to this population. The efficacy of a third dose of the SARS-CoV-2 vaccine in SOT recipients varies significantly across studies according to the transplant type and vaccine platform. It is important to consider that not all SOT recipients are the same. Liver transplant recipients (LTR) generally account for a lesser degree of immunosuppression compared to kidney and heart recipients. Thus, the data available on LTR who were immunized with SARS-CoV-2 vaccines needs to be examined separately from other SOT recipients.
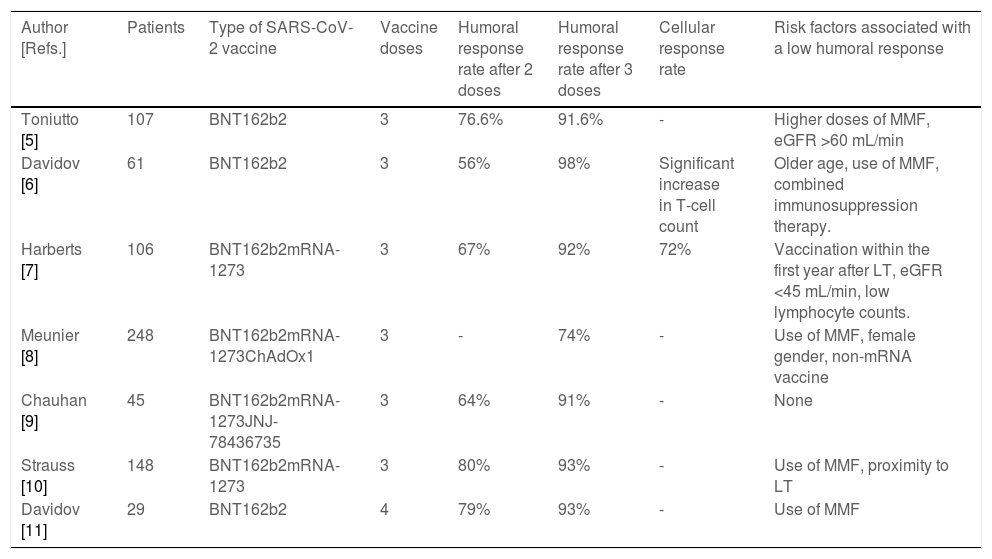
Data on the humoral response rates in LTR to different platforms of SARS-CoV-2 ranged from 17% to 87% after two doses, being significantly lower than in the general population [3,4]. The reduced immune response was more frequent in patients who received mycophenolate mofetil (MMF), those with a shorter time to transplantation and in patients with older age or with renal impairment [3,5–11]. On the other hand, the improved immune response was described in patients who received heterologous adenovirus-vector/mRNA regimens [12]. Recent studies have demonstrated a robust efficacy of a third SARS-CoV-2 vaccination in LTR (Table 1). More than half of those patients who did not have a humoral response after 2 vaccine doses developed a response after a third dose. The exact antibody level required to protect against infection with variants of concern is unknown, but some authors have suggested that the degree of protection against infections and severe disease courses increases with rising antibody levels [13]. In LTR antibody levels remain significantly lower than in healthy controls and roughly 10%–20% of LTR did not display humoral response after a third shot, which suggests that this patient population remains at risk for infection (Table 1). Of note, serious adverse events or acute rejection episodes after additional vaccine boosters continue to be rare.
Studies evaluating the use of booster vaccine doses against SARS-CoV-2 infection.
| Author [Refs.] | Patients | Type of SARS-CoV-2 vaccine | Vaccine doses | Humoral response rate after 2 doses | Humoral response rate after 3 doses | Cellular response rate | Risk factors associated with a low humoral response |
|---|---|---|---|---|---|---|---|
| Toniutto [5] | 107 | BNT162b2 | 3 | 76.6% | 91.6% | - | Higher doses of MMF, eGFR >60 mL/min |
| Davidov [6] | 61 | BNT162b2 | 3 | 56% | 98% | Significant increase in T-cell count | Older age, use of MMF, combined immunosuppression therapy. |
| Harberts [7] | 106 | BNT162b2mRNA-1273 | 3 | 67% | 92% | 72% | Vaccination within the first year after LT, eGFR <45 mL/min, low lymphocyte counts. |
| Meunier [8] | 248 | BNT162b2mRNA-1273ChAdOx1 | 3 | - | 74% | - | Use of MMF, female gender, non-mRNA vaccine |
| Chauhan [9] | 45 | BNT162b2mRNA-1273JNJ-78436735 | 3 | 64% | 91% | - | None |
| Strauss [10] | 148 | BNT162b2mRNA-1273 | 3 | 80% | 93% | - | Use of MMF, proximity to LT |
| Davidov [11] | 29 | BNT162b2 | 4 | 79% | 93% | - | Use of MMF |
eGFR, estimated glomerular filtration rate; LT, liver transplant; MMF, mycophenolate mofetil.
Investigators are highly reliant on humoral response and lack other indicators that are also suggestive of immunity such as neutralizing antibody assay and cellular response. Neutralizing level have been associated with immune protection and T cells reduce viral loads and disease. These outcomes are also of vital importance and usually not reported probably because they are expensive assays and the quantification of virus-specific T-cell immune responses is technically more complex than performing serological analyses. Consequently, limited data have been published regarding the role of T cells in the protection against SARS-CoV-2 infection in SOT. Harberts et al. described and increased proportion of LTR with positive T-cell response from 32% to 72% after the third mRNA vaccine dose [7]. Likewise, Davidov et al. reported that neutralizing antibody levels increased significantly after the third dose compared to levels measured immediately before its administration [6].
Many variables were associated with an increased risk for low antibody levels. However, MMF would appear to be the main determinant of vaccination failure. A recent meta-analysis evaluated the relationship between MMF and seroconversion after SARS-CoV-2 immunization in SOT recipients. When the analysis was restricted to studies performed in LTR, the negative effect of MMF was consistent (OR=0.46; 95%CI 0.31–0.68; p<.001) [14]. In some studies, a dose-dependent impairment of the humoral response was observed, particularly when the daily dose of MMF was higher than 1000 mg per day [12,14]. The detrimental effects of an MMF-containing immunosuppressive regimen in the vaccination induced humoral immune response was also described in SOT recipients after vaccination against the influenza virus [15]. This can be explained by the MMF inhibition of T- and B-cell proliferation by depleting guanosine nucleotides through inhibition of the enzyme inosine monophosphate dehydrogenase, which is expressed preferentially in activated lymphocytes [16]. The reduction or discontinuation of MMF to increase vaccine response is still under debate. Meunier et al. evaluated the impact of temporary discontinuation of MMF on vaccine response in 10 non-responding LTR. The authors showed that a complete MMF withdrawal for 1 to 4 weeks before vaccination was safe and allowed patients to achieve seroconversion [17]. Future studies replicating these promising results are expected.
Different reports suggested a rapid decline in levels of antibodies after 4-5 months onwards compared to immunocompetent individuals [6]. Thus, immunosuppressed patients are prioritized to receive a booster vaccine dose. Data on the effectiveness of the fourth vaccine dose among LTR is very limited. Harberts et al. also described a seroconversion of 60% (3/5) among previous non-responders to the third vaccine [7]. Later, Davidov et al. reported an improved immune response after the fourth BNT162b2 vaccine dose improving humoral response from 79% to 93% [11]. In line with previous observations, this study found a significant association between immune response and MMF administration. Since antiviral responses to SARS-CoV-2 vaccines in SOT recipients are attenuated, additional strategies such as monoclonal antibody pre-exposure prophylaxis has been recommended. The most widely used monoclonal antibody in SOT is the long-acting combination tixagevimab/cilgavimab. This therapy has been associated with a lower risk of SARS-CoV-2 infection in unvaccinated individuals and in SOT recipients during the Omicron wave. Importantly, tixagevimab/cilgavimab showed a slightly higher incidence of serious cardiovascular events [18]. This monoclonal antibody combination arises as an interesting alternative in those patients who did not develop immune response to SARS-CoV-2 vaccination. We look forward to a more detailed understanding of the protective role and adverse events of tixagevimab/cilgavimab. However, the cost of this combination might limit its accessibility in Latin America.
We still need to evaluate different approaches to prevent or diminish COVID-19 infection in LTR. As this population appears to remain at risk for infection after 3 or 4 doses, investigations regarding the efficacy and timing of future doses should be considered. It is therefore important to be able to predict non-response to vaccination so that extra modifiable risk factors can be addressed, or extra protection administered. In the meantime, patients and healthcare providers should remain vigilant regarding exposure to infection.