Infection with hepatitis C virus (HCV) genotype (G)5 or G6 is relatively uncommon worldwide, with data on the efficacy of peginterferon/ribavirin (PegI-FN/RBV) treatment limited, and sometimes contradictory compared with other HCV genotypes.1–3 G5 is predominantly found in South Africa, with local clusters reported in France, Spain, Syria, Belgium and Canada, while G6 has been reported in Asian countries and patients of Asian origin in the US and Canada.4 Here, we report data from the 25 G5 and G6 patients from the “real world” PROPHESYS study5 receiving PegIFN alfa-2a (40KD) (PEGASYS®, Roche, Basel, Switzerland) plus RBV.
Prophesys was a prospective, non-interventional study of chronic HCV patients receiving treatment according to country-specific requirements, comprising three cohorts of patients enrolled in 19 countries.5 The primary analysis population (N = 7,163) included treatment-naive HCV mono-infected patients who received ≥ 1 dose of PegIFN/RBV, with baseline HCV RNA ≥ 50 IU/mL and ≥ 1 post-baseline HCV RNA test result.
Patients with G5 (n = 15) had a mean age of 55.2 years and 3/14 assessed for liver fibrosis (21%) were cirrhotic. They were predominantly male (73%) and White (87%), with high baseline viral load (median [range] HCV RNA 6.4 [5.4-7.0] log10 IU/mL; > 800,000 IU/mL in 87%). G6 patients (n =10) had a mean age of 48.4 years and 4/7 assessed (57%) were cirrhotic. They were predominantly male (70%) and Asian (90%), with high baseline viral load (median [range] HCV RNA 6.3 [3.7-7.7] log10 IU/mL; > 800,000 IU/mL in 70%). Mean treatment duration was 46 and 41 weeks in G5 and G6 patients, respectively (with 93% and 90% receiving > 80% of PegI-FN alfa-2a/RBV target doses).
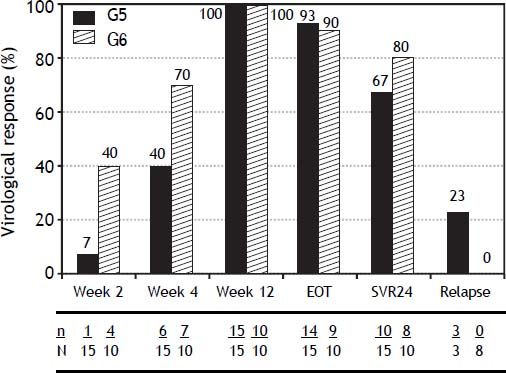
SVR24 rates (HCV RNA < 50 IU/mL 24 weeks after end of treatment [EOT]) were 67% and 80% in G5 and G6 patients, respectively (Figure 1). Relapse within 24 weeks after EOT (in patients with EOT virological response [VR] and sufficient post-EOT data) was reported in 3 G5 patients (23%) and in no G6 patients. The positive predictive value (PPV) of VR by week 4 for achieving SVR24 was 100% (6/6; 95% CI 54-100) in G5 patients and 86% (6/7; 95% CI 42-100) in G6 patients.
SVR24 rates in G5 and G6 patients (67% and 80%) were comparable to those seen in G2 and G3 (71 and 61%, respectively) and higher than G1 and G4 (42 and 41%) patients in PROPHESYS.5 A similar pattern was recently reported in Germany, where SVR24 rates in G5 and G6 patients (58 and 59%) were comparable to G2 and G3 patients (60 and 58%) and superior to G1 and G4 patients (42 and 44%).3 In contrast, a meta-analysis of two prospective clinical trials reported SVR24 rates in G5 patients (55%) more similar to those seen in G1 (50%) than G2 and G3 patients (75%).1
PegIFN/RBV is currently the standard treatment option for G5 and G6 patients, and despite these and other studies being limited by the small numbers of G5/6 patients available, data support the use of PegIFN/RBV in these patients. The high PPV of VR by week 4 for achieving SVR24 in G5/6 p atients supports the potential use of viral kinetics in treatment decisions.
Abbreviations- •
EOT: end of treatment.
- •
G: genotype.
- •
HCV: hepatitis C Virus.
- •
PegEFN: peginterferon.
- •
PPV: positive predictive value.
- •
RBV: ribavirin.
- •
SVR: sustained virological response.
- •
VR: virological response.
This study was supported by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland.