Biliary atresia (BA) is characterized by rapid progression of fibrosis with no definite causes. Histopathological findings have been extensively described, but very few studies have assessed temporal changes in BA. Understanding these short-term changes and their relationship with fibrosis progression could have an impact on ameliorating rapid fibrogenesis. We aimed to study the relationship between temporal histopathological changes and fibrosis progression in BA within a short time interval.
Patients and MethodsForty-nine infants with BA who underwent Kasai portoenterostomy, a diagnostic liver biopsy, and an intraoperative liver biopsy were recruited. Histopathological characteristics of the two biopsies were examined. Temporal histopathological changes were assessed by comparing the two types of biopsies. Correlation of temporal changes in fibrosis with age, interval between biopsies, laboratory profiles, and temporal histopathological changes were studied.
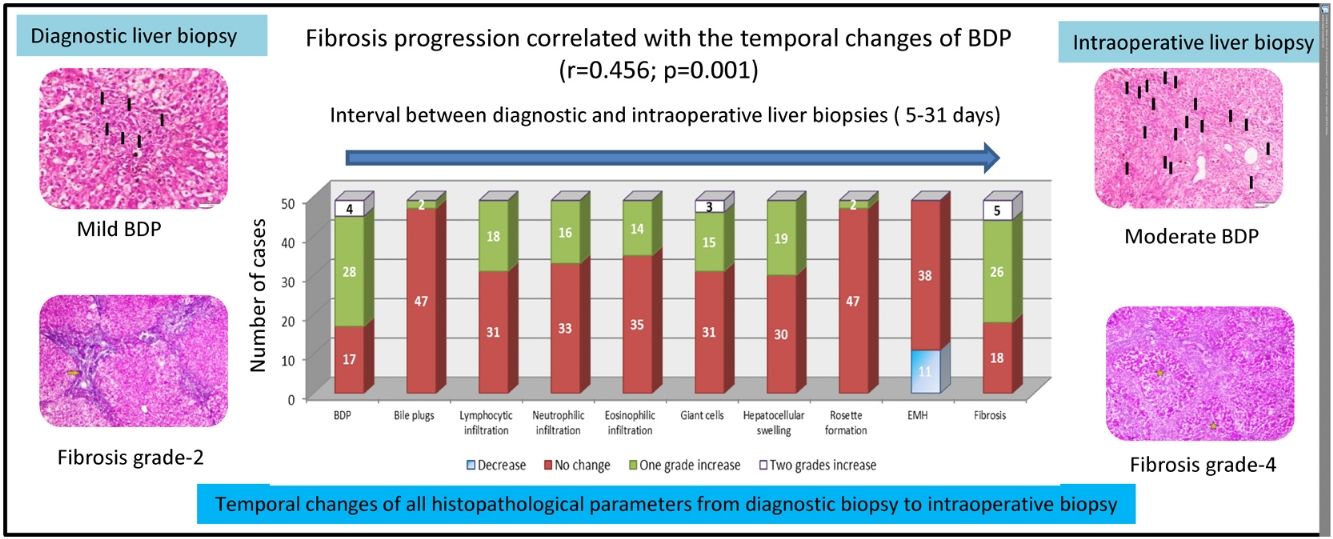
ResultsIn the univariate analysis, bile ductular proliferation (BDP), portal infiltrate, giant cells, hepatocellular swelling, and fibrosis showed significant temporal changes within a short interval (5–31 days). BDP and fibrosis showed the most frequent increase in their grades (32/49 and 31/49 cases, respectively). In the multivariate analysis, BDP was the only independent pathological feature showing a significant temporal increase (p = 0.021, 95% confidence interval: 1.249–16.017). Fibrosis progression was correlated with temporal changes in BDP (r = 0.456, p = 0.001), but not with age (p = 0.283) or the interval between the biopsies (p = 0.309).
ConclusionsFibrosis in BA progresses rapidly and is significantly correlated with BDP. Assessment of targeting BDP as an adjuvant medical therapy is recommended.
Biliary atresia (BA) is the commonest cause of neonatal cholestasis. It is associated with a unique feature, namely progressive fibrosis. In most cases, even after timely Kasai portoenterostomy (KP) with successful biliary drainage, fibrosis progresses with the development of cirrhosis and portal hypertension [1]. Mechanisms underlying this rapid fibrosis progression are unknown.
A younger infant with BA could exhibit a more advanced grade of fibrosis than an older infant [2]. This suggests that fibrosis progression is not related to aging alone and there are driving forces behind the different grades of fibrosis among different cases.
The word fibrosis is used to describe the recovery process of an injured liver. Progressive fibrosis leads to harmful effects with the development of portal hypertension and its hazardous consequences [3]. It is well known that there is a rapid progression to obliterative fibrosis in BA patients. However, preventing fibrosis progression that occurs even after successful KP remains challenging [4]. Investigating early histopathological changes in BA that might be related to rapid progression of fibrosis could have an impact on ameliorating this rapidly progressive disease.
Histopathological criteria of BA are well studied. Most of the studies have shown that bile ductular proliferation (BDP), bile plugs, and portal fibrosis are the most important pathological features of BA along with different grades of other pathological features such as portal cellular infiltrates, giant cells, and hepatocellular swelling [5,6].
Some previous [7] and recent [8] studies have demonstrated the prognostic significance of various histopathological features such as BDP in BA. However, dynamic changes in these histopathological criteria and their relationship with progression of fibrosis have not been adequately recognized. The present study aimed to assess the relationship between temporal histopathological changes and fibrosis progression in BA within a short time interval.
2Materials and methods2.1Study populationThis observational prospective cohort study included 98 specimens of liver tissue from 49 infants with BA, which included two sequential specimens from each patient (a preoperative diagnostic liver biopsy specimen and an intraoperative liver biopsy specimen). The patients were recruited consecutively from June 2015 to May 2018 from the Department of Pediatric Hepatology, Gastroenterology, and Nutrition.
Within the duration of the study, all infants diagnosed with BA were recruited. Those who had undergone a diagnostic (first) and an intraoperative (second) liver biopsy were included in the study. The exclusion criteria were (1) inadequate liver biopsy [9], (2) loss of liver tissue during the processing procedures for staining, and (3) cytomegalovirus positive cases.
Preoperative diagnosis of BA was made according to a variety of criteria after full history taking, thorough clinical examination, routine investigations, and liver biopsy, as reported by us previously in the BA scoring system [10]. The diagnosis of BA was confirmed by operative cholangiography and/or intraoperative laparotomy findings immediately before proceeding to KP.
All BA cases included in the present study were isolated forms of BA. One case of biliary atresia splenic malformation was excluded due to inadequate tissue in the diagnostic liver biopsy. One case of cystic variant of BA was excluded due to cytomegalovirus positivity. Thus, the study cohort consisted of patients with isolated, typical variant of BA [4].
In compliance with the 1975 Declaration of Helsinki and its later amendments, approval for this study was obtained from the Research Ethics Committee of the National Liver Institute. Signed informed consents were obtained from the parents of all patients.
2.2Biochemical, hematological, and ultrasonographic evaluationLiver function tests (total and direct bilirubin, total proteins, albumin, alanine transaminase, aspartate transaminase, γ-glutamyl transpeptidase, and alkaline phosphatase), determination of international normalized ratio, and complete blood count were performed for each infant. Abdominal ultrasound and Doppler study were performed for all cases, as described by us previously [11]. All of these investigations were performed at the time of the diagnostic liver biopsy.
2.3Liver biopsyAll recruited patients underwent an ultrasound-guided diagnostic liver biopsy (first liver biopsy) using a Tru-cut needle at the initial assessment and an intraoperative wedge biopsy (second liver biopsy) during KP. All intraoperative biopsies were sampled 5 mm deeper to the capsule to avoid the subcapsular fibrosis and to obtain tissue comparable to that sampled by the Tru-cut biopsy [12].
2.4Histopathological evaluationFor histopathological evaluation, tissue sections were stained with hematoxylin and eosin, diastase periodic acid-Schiff, orcein, and Masson’s trichrome stains. Specimens containing ten or more portal tracts were considered adequate [9].
All specimens were assessed for the presence of (1) BDP, (2) bile plugs, (3) portal infiltrate with lymphocytes, neutrophils, and eosinophils, (4) giant cell transformation of hepatocytes, (5) hepatocyte swelling, (6) rosette formation, and (7) extramedullary hematopoiesis (EMH).
BDP, giant cell transformation of hepatocytes, and bile plugs were graded according to the method reported by Lee and Looi [5]. The other features were graded based on the experience of our pathology department with the criteria for each grade clearly defined to avoid subjective assessment.
BDP was graded as 1) none: the average number of bile ductules per portal tract <5, 2) mild: the average number of bile ductules per portal tract between 5 and 9, 3) moderate: the average number of bile ductules per portal tract ≥10, or 4) marked: elongated, attenuated, or angulated bile ductules in addition to proliferation (average number of bile ductules per portal tract ≥10). Presence of giant cells was reported as none, giant cells around the central vein, or diffuse. Bile plugs were reported as absent or present.
Lymphocytic infiltration in the portal region was reported as none (presence of up to four normal resident lymphocytes in the portal tract), mild (presence of a large number of lymphocytes in the portal tract without the presence of lymphoid aggregates), or moderate to marked (presence of a large number of lymphocytes in the portal tract with the presence of lymphoid aggregates). Neutrophilic infiltration in the portal region was reported as 1) absent or mild (presence of a few neutrophils around some bile ducts) or 2) moderate to marked (presence of a large number of neutrophils around some bile ducts). Eosinophilic infiltration in the portal region was reported as 1) absent or mild (presence of a few eosinophils around some bile ducts) or 2) moderate to marked (presence of a large number of eosinophils around some bile ducts). Hepatocyte swelling was reported as 1) none, 2) mild/focal, or 3) periportal/diffuse. EMH and rosette formation were reported as absent or present.
Liver fibrosis was classified into five grades [13]. Grade 0 indicated absence of fibrosis or fibrous expansion of some portal areas, grade 1 indicated fibrous expansion of most of the portal areas, grade 2 indicated focal porto-portal bridging, grade 3 indicated marked bridging, and grade 4 indicated cirrhosis.
Temporal histopathological changes were assessed by comparing the diagnostic and the intraoperative liver biopsies.
All histopathological findings were interpreted by two senior pathologists who were blinded to the diagnosis of the patients or the type of biopsy. All pathological criteria were assessed three times, one month apart and median values were reported. Intraobserver and interobserver kappa values for different pathological parameters were 0.831–0.886 and 0.795–0.827, respectively. For the histopathological findings that showed no concordance between the two pathologists, a shared reassessment was performed.
2.5Statistical analysisQuantitative parametric data were expressed as mean ± standard deviation and non-parametric data were expressed as median (minimum–maximum). Qualitative data were presented as number (percentage) of individuals with a particular condition. Chi-squared test or Fisher’s exact test was used to determine the level of significance for the qualitative data. Correlation of temporal changes in fibrosis with other parameters was tested using Spearman’s test. Stepwise regression analysis was performed to determine the independent temporal histopathological changes. The kappa coefficient was calculated to measure the intraobserver and interobserver agreement. The value of the kappa coefficient was used to denote the following agreement categories: excellent (≥0.80), good (0.60–0.79), moderate (0.40–0.59), poor (0.20–0.39), and very poor (<0.20). A p-value of less than 0.05 was used to indicate statistically significant results. SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analysis.
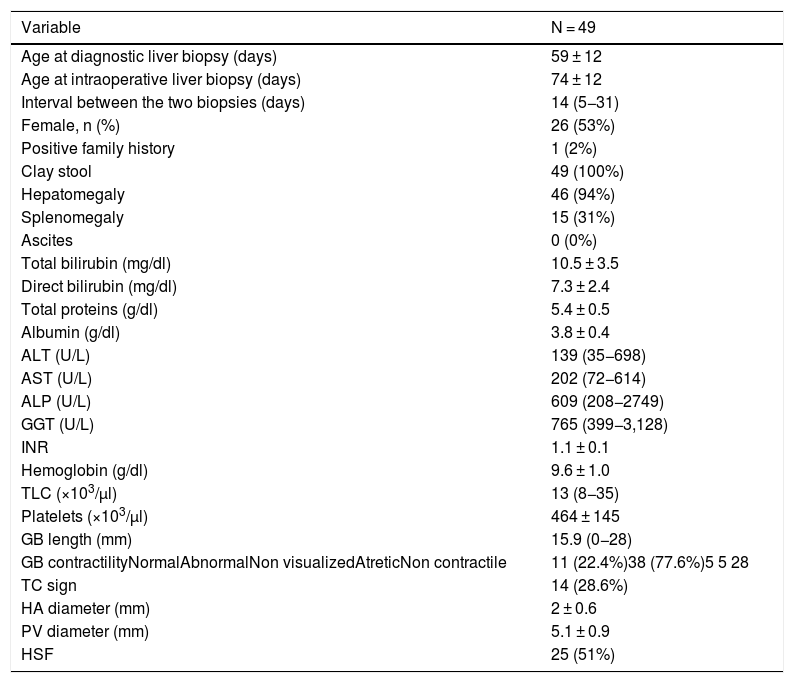
3Results3.1Demographic, clinical, laboratory, and radiologic data of the groupThe mean age at diagnostic liver biopsy was 59 ± 12 days (range: 33–98 days), while the mean age at KP and the intraoperative liver biopsy was 74 ± 12 days (range: 49–117 days). The interval between the two liver biopsies ranged from 5 days to 31 days with a median of 14 days. Females constituted 53% of the cases and one case (2%) had a positive family history. All cases exhibited clay-colored stools, 94% of the cases had hepatomegaly, and 77.6% of the cases exhibited abnormal gall bladder contractility on ultrasound evaluation. Splenic malformation syndrome was not reported in any of the cases. Other clinical, laboratory, and radiological data are presented in Table 1.
Demographic, clinical, laboratory, and radiologic parameters at the timing of diagnostic liver biopsy.
| Variable | N = 49 |
|---|---|
| Age at diagnostic liver biopsy (days) | 59 ± 12 |
| Age at intraoperative liver biopsy (days) | 74 ± 12 |
| Interval between the two biopsies (days) | 14 (5−31) |
| Female, n (%) | 26 (53%) |
| Positive family history | 1 (2%) |
| Clay stool | 49 (100%) |
| Hepatomegaly | 46 (94%) |
| Splenomegaly | 15 (31%) |
| Ascites | 0 (0%) |
| Total bilirubin (mg/dl) | 10.5 ± 3.5 |
| Direct bilirubin (mg/dl) | 7.3 ± 2.4 |
| Total proteins (g/dl) | 5.4 ± 0.5 |
| Albumin (g/dl) | 3.8 ± 0.4 |
| ALT (U/L) | 139 (35−698) |
| AST (U/L) | 202 (72−614) |
| ALP (U/L) | 609 (208−2749) |
| GGT (U/L) | 765 (399−3,128) |
| INR | 1.1 ± 0.1 |
| Hemoglobin (g/dl) | 9.6 ± 1.0 |
| TLC (×103/µl) | 13 (8−35) |
| Platelets (×103/µl) | 464 ± 145 |
| GB length (mm) | 15.9 (0−28) |
| GB contractilityNormalAbnormalNon visualizedAtreticNon contractile | 11 (22.4%)38 (77.6%)5 5 28 |
| TC sign | 14 (28.6%) |
| HA diameter (mm) | 2 ± 0.6 |
| PV diameter (mm) | 5.1 ± 0.9 |
| HSF | 25 (51%) |
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BA, biliary atresia; cm, centimeter; dl, deciliter; g, gram; GB, gall bladder; GGT, γ-glutamyltransferase; HA, hepatic artery; HSF, hepatic subcapsular flow; INR, international normalized ratio; Kg, kilogram; mg, milligram; µl, microliter; PT, prothrombin time; PTT, partial thromboplastin time; PV, portal vein; TC, triangular cord sign; TLC: total leucocyte count.
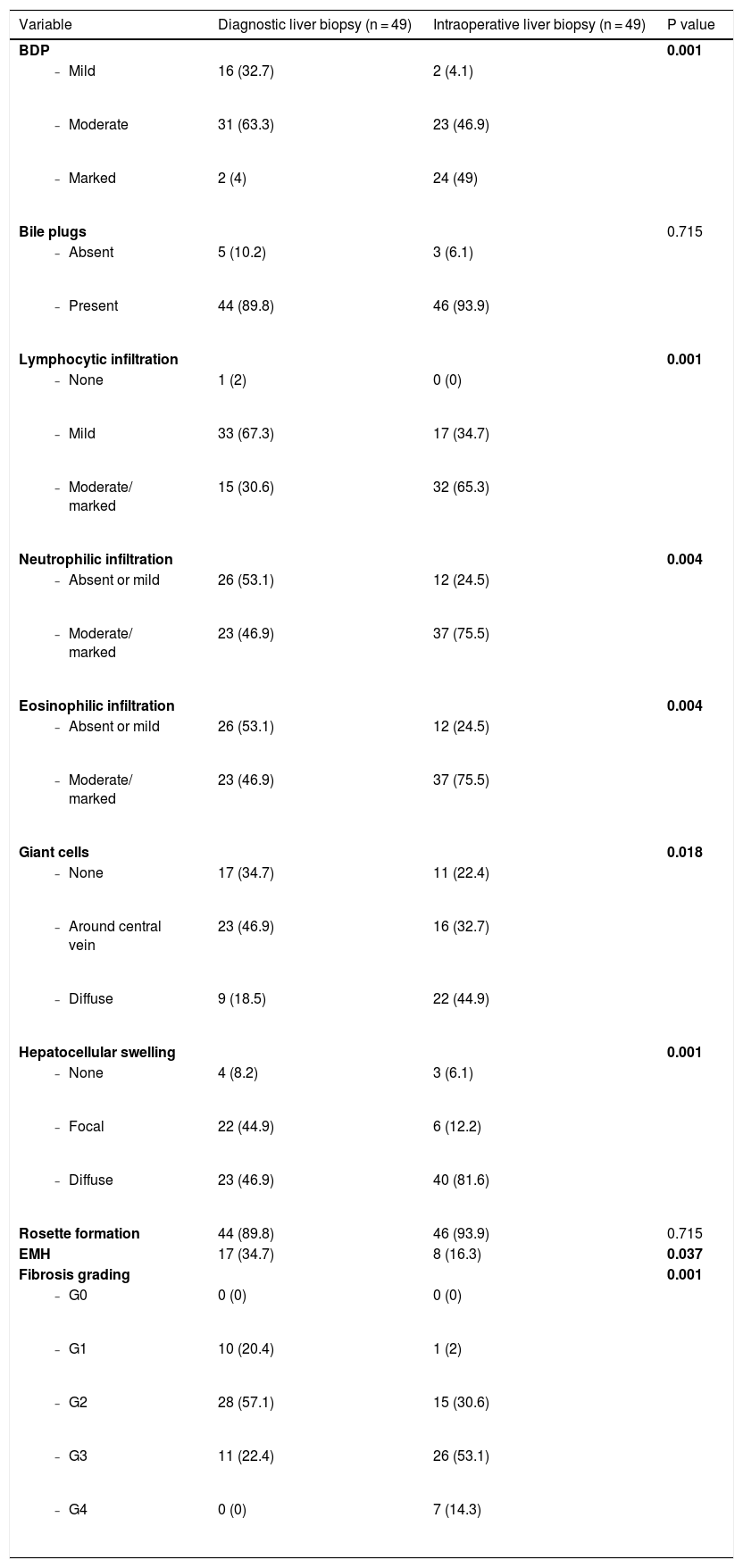
BDP was observed in all liver biopsy specimens (100%) irrespective of the type of biopsy. Moreover, all biopsies (100%) showed different grades of fibrosis, with established cirrhosis in 14.3% of the cases at the time of KP. Both bile plugs and rosette formation were also prevalent histopathological findings. They were observed in 89.8% of the cases in the initial liver biopsy and in 93.9% of the cases in the intraoperative liver biopsy. On the other hand, EMH was present in a few cases either in the first or in the second liver biopsy (34.7% and 16.3% of the cases, respectively). Additional histopathological findings are presented in Table 2.
Histopathological changes of diagnostic and intraoperative liver biopsies.
| Variable | Diagnostic liver biopsy (n = 49) | Intraoperative liver biopsy (n = 49) | P value |
|---|---|---|---|
| BDP | 0.001 | ||
| 16 (32.7) | 2 (4.1) | |
| 31 (63.3) | 23 (46.9) | |
| 2 (4) | 24 (49) | |
| Bile plugs | 0.715 | ||
| 5 (10.2) | 3 (6.1) | |
| 44 (89.8) | 46 (93.9) | |
| Lymphocytic infiltration | 0.001 | ||
| 1 (2) | 0 (0) | |
| 33 (67.3) | 17 (34.7) | |
| 15 (30.6) | 32 (65.3) | |
| Neutrophilic infiltration | 0.004 | ||
| 26 (53.1) | 12 (24.5) | |
| 23 (46.9) | 37 (75.5) | |
| Eosinophilic infiltration | 0.004 | ||
| 26 (53.1) | 12 (24.5) | |
| 23 (46.9) | 37 (75.5) | |
| Giant cells | 0.018 | ||
| 17 (34.7) | 11 (22.4) | |
| 23 (46.9) | 16 (32.7) | |
| 9 (18.5) | 22 (44.9) | |
| Hepatocellular swelling | 0.001 | ||
| 4 (8.2) | 3 (6.1) | |
| 22 (44.9) | 6 (12.2) | |
| 23 (46.9) | 40 (81.6) | |
| Rosette formation | 44 (89.8) | 46 (93.9) | 0.715 |
| EMH | 17 (34.7) | 8 (16.3) | 0.037 |
| Fibrosis grading | 0.001 | ||
| 0 (0) | 0 (0) | |
| 10 (20.4) | 1 (2) | |
| 28 (57.1) | 15 (30.6) | |
| 11 (22.4) | 26 (53.1) | |
| 0 (0) | 7 (14.3) |
Bolding of p value indicates statistical significance.
Abbreviations: BA: biliary atresia; BDP: bile ductular proliferation; EMH: Extramedullary hematopoiesis; G: grade.
We performed a univariate analysis for temporal histopathological changes in BA by comparing the pathological findings in the first liver biopsy with those in the second liver biopsy, with an interval ranging from 5 days to 31 days (median: 14 days). There was a significant increase in BDP (p = 0.001), portal tract cellular infiltrate (lymphocytic, neutrophilic, and eosinophilic: p = 0.001, p = 0.004, and p = 0.004, respectively), giant cells (p = 0.018), hepatocellular swelling (p = 0.001), and fibrosis (p = 0.001) (Table 2 and Fig. 1). On the other hand, EMH showed a significant decrease (p = 0.037) with no significant changes in bile plugs and rosette formation (Table 2).
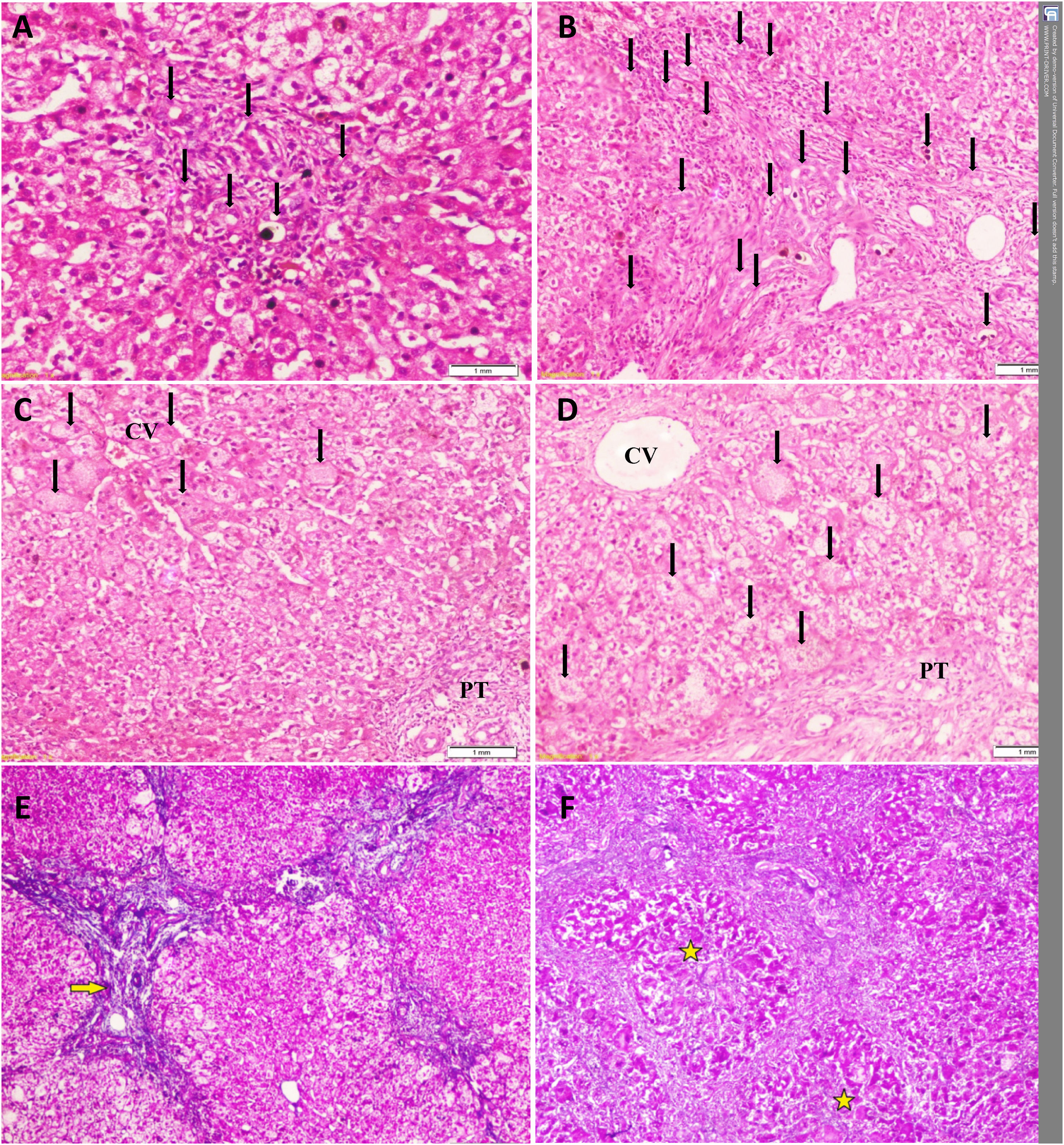
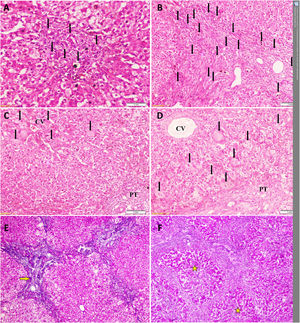
Temporal histopathological changes in biliary atresia.
A: Mild bile ductular proliferation (BDP) (arrows) and C: giant cells (arrows) around the CV in the first liver biopsy of a 61-day-old infant.
B: Moderate BDP and D: giant cells with diffuse distribution (arrows) in the second liver biopsy of the same infant at an age of 79 days.
E: Grade-2 fibrosis (arrow) in the first liver biopsy of another 71-day-old infant that progressed to (F) grade-4 fibrosis (stars) in his second liver biopsy at an age of 82 days Slides: A to D (Hematoxylin and eosin, ×200), E and F (Masson’s trichrome stain, ×40)
CV: central vein, PT: portal tract.
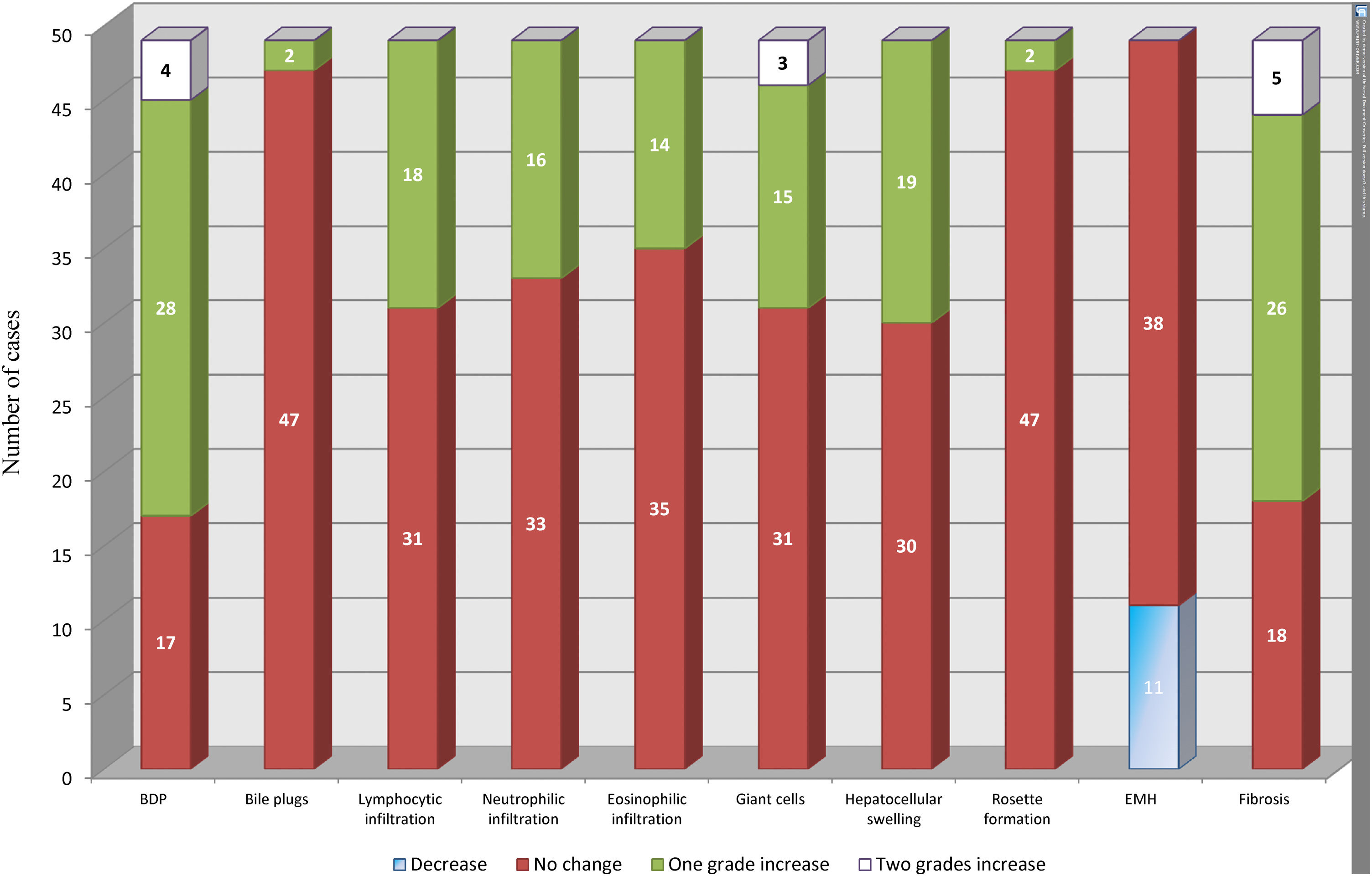
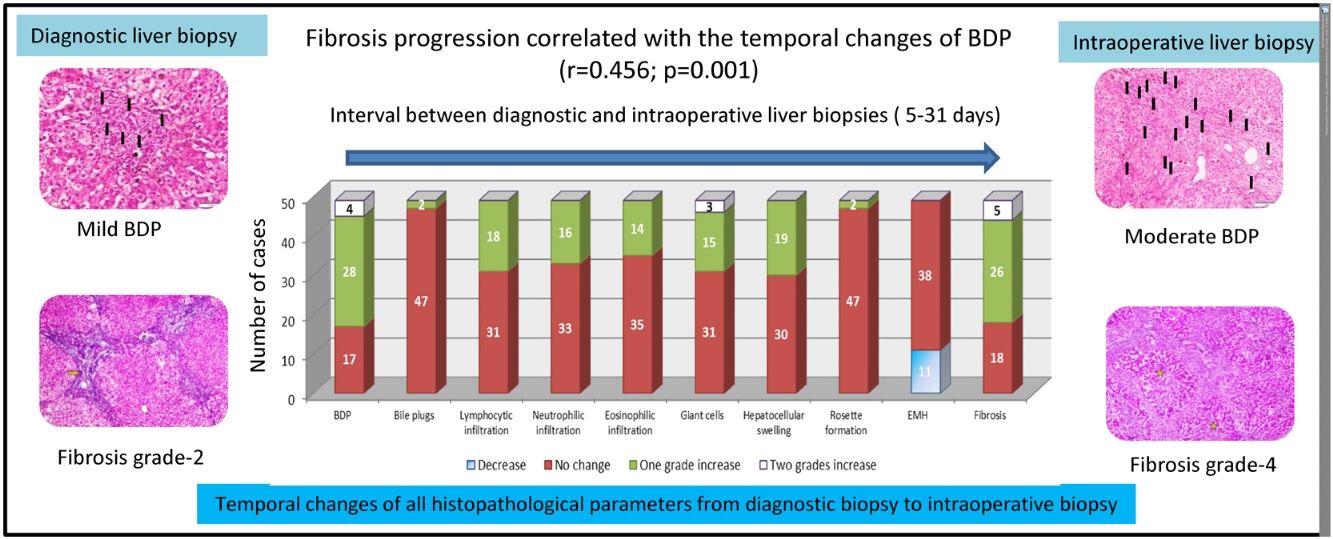
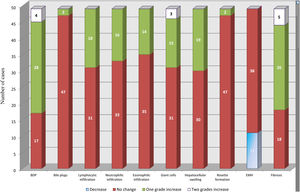
BDP showed a temporal increase from the diagnostic biopsy to the intraoperative biopsy by one grade in 28 cases and by two grades in four cases. This was followed by fibrosis, which showed a temporal increase by one grade in 26 cases and by two grades in five cases. No change was observed in the grade of fibrosis in 18 cases. Temporal changes in the other histopathological findings are depicted in Fig. 2.
Temporal changes in all histopathological parameters from the diagnostic biopsy to the intraoperative biopsy.
BDP showed the highest temporal change with an increase by one grade in 28 cases and an increase by two grades in four cases. This was followed by an increase in fibrosis by one grade in 26 cases and by two grades in five cases. All other histopathological parameters showed different temporal changes.
BDP: bile ductular proliferation, EMH: extramedullary hematopoiesis
In the multivariate analysis, BDP was the only histopathological feature that showed a significant difference between the first and the second liver biopsies (p = 0.021, 95% confidence interval: 1.249–16.017). It showed a significant increase in the grades from 4% in the diagnostic liver biopsy to 49% in the intraoperative liver biopsy (Table 2).
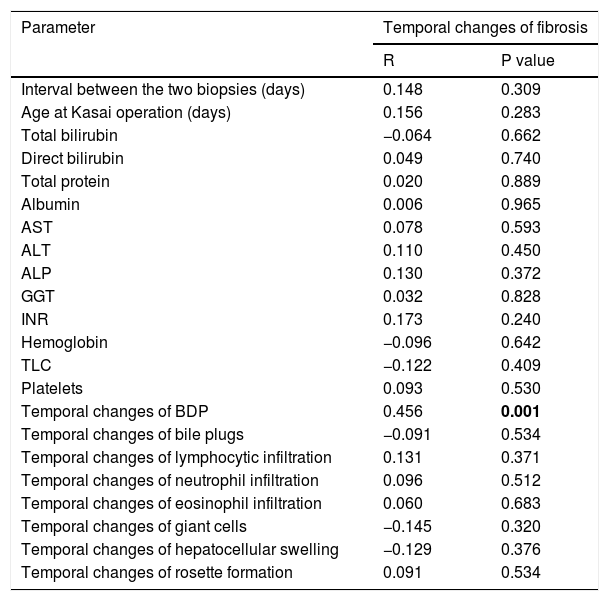
3.4Relationship of temporal changes in fibrosis with other parametersTemporal changes in fibrosis showed a significant positive correlation with temporal changes in BDP (r = 0.456, p = 0.001). On the other hand, they showed no correlation with the interval between the two liver biopsies (r = 0.148, p = 0.309) or with the age of the infants at KP (r = 0.156, p = 0.283). Moreover, temporal changes in fibrosis showed no correlation with other laboratory parameters under study and with histopathological temporal changes (p > 0.05 for all) (Table 3).
Correlation of temporal changes of fibrosis with other parameters studied.
| Parameter | Temporal changes of fibrosis | |
|---|---|---|
| R | P value | |
| Interval between the two biopsies (days) | 0.148 | 0.309 |
| Age at Kasai operation (days) | 0.156 | 0.283 |
| Total bilirubin | −0.064 | 0.662 |
| Direct bilirubin | 0.049 | 0.740 |
| Total protein | 0.020 | 0.889 |
| Albumin | 0.006 | 0.965 |
| AST | 0.078 | 0.593 |
| ALT | 0.110 | 0.450 |
| ALP | 0.130 | 0.372 |
| GGT | 0.032 | 0.828 |
| INR | 0.173 | 0.240 |
| Hemoglobin | −0.096 | 0.642 |
| TLC | −0.122 | 0.409 |
| Platelets | 0.093 | 0.530 |
| Temporal changes of BDP | 0.456 | 0.001 |
| Temporal changes of bile plugs | −0.091 | 0.534 |
| Temporal changes of lymphocytic infiltration | 0.131 | 0.371 |
| Temporal changes of neutrophil infiltration | 0.096 | 0.512 |
| Temporal changes of eosinophil infiltration | 0.060 | 0.683 |
| Temporal changes of giant cells | −0.145 | 0.320 |
| Temporal changes of hepatocellular swelling | −0.129 | 0.376 |
| Temporal changes of rosette formation | 0.091 | 0.534 |
Bolding of P value means statistical significance.
Laboratory parameters are those at the timing of the diagnostic liver biopsy.
Abbreviations: ALT: alanine aminotransferase; ALP: alkaline phosphatase; AST: aspartate aminotransferase; BDP: bile ductular proliferation; GGT: gamma glutamyl transferase; INR: international normalized ratio; TLC: total leucocyte count.
Even with successful biliary drainage, most of the operated BA cases exhibit progression of fibrosis at variable rates with the development of portal hypertension [1]. Since fibrosis is a significant determinant of morbidity even in BA cases operated in a timely manner [13], studying the factors that are related to fibrosis progression in this disease is of utmost importance.
In the present study, different grades of liver fibrosis were observed in all cases in the early stages of BA (59 ± 12 days). Fibrosis progressed after a short duration (5–31 days, median: 14 days) to significantly higher grades (p = 0.001). Kim et al. [14] reported that in BA, liver fibrosis may progress rapidly and even cirrhosis may ensue at as early as 2 months of age. Rastogi et al. [15] and Russo et al. [13] also reported high grades of fibrosis in the early stage of BA. In the present study, 14.3% of the cases progressed to cirrhosis at the time of KP.
The present study and other previous studies showed that a high grade of fibrosis is a feature of BA. Interestingly, fibrosis progression was not correlated with the age of the infant at KP or with the interval between the two biopsies (p = 0.283 and p = 0.309, respectively). Thus, fibrosis progression in BA is not related to time alone. Unique factors could drive this rapid progression that could even lead to a more advanced grade of fibrosis in a younger infant when compared with that in an older infant.
BDP was observed in all cases of BA (in the diagnostic liver biopsy as well as in the subsequent intraoperative liver biopsy). With progression of BA from early to late stages, there was a significant increase in the grades of BDP (p = 0.001) and increased inflammatory activity in the form of cellular infiltrate, hepatocellular swelling, and rosette formation. All of these histopathological changes are expected to encourage fibrogenesis and its progression [16–19].
Understanding the dynamic histopathological changes in BA that are suggested to have a relationship with fibrogenesis [2,20–22] might have a therapeutic implication in interventions to prevent or to ameliorate this unique rapid process.
It is well known that BDP has a prognostic value in BA [7,8]. The novel factor in the present study is the description of the changes (decrease, no change, increase) in the histopathological criteria of BA within a short time interval. Moreover, testing the correlation of these temporal changes with fibrosis progression is also a novel factor compared to testing at a cross sectional level, as reported in previous studies.
In the present study, we observed a significant temporal increase in BDP, cellular (lymphocytes, neutrophils, and eosinophils) infiltrate, and fibrosis within a short time interval (5–31 days) in the univariate analysis. Fibrosis and BDP increased by more than 60%. However, in the multivariate regression analysis, BDP was the only histopathological finding that showed a significant temporal increase (p = 0.021, 95% confidence interval: 1.249–16.017).
Zhang et al. [2] reported a temporal increase in BDP, inflammation, and fibrosis grade from the intraoperative liver biopsy to the postoperative liver biopsy. They also reported that the severity of fibrosis was positively correlated with the severity of BDP.
In the present study, the finding that BDP was the only independent factor showing a temporal histopathological increase in BA cases within a short time interval puts this histopathological feature in the scope of interest in this devastating disease.
Previous studies [2,23,24] have proposed that portal fibrosis in cholestatic liver diseases appears to be induced by reactive cholangiocytes forming the proliferating bile ductules of BDP. It has been shown that ductal epithelium can express proteins that attract and activate hepatic stellate cells, leading to collagen deposition. Many studies proved the relationship between BDP and the activation of these hepatic stellate cells [25]. Clouston et al. [25], Fabris et al. [26] and Zhang et al. [27] reported that the severity of fibrosis was significantly correlated with the grade of BDP.
The proliferating bile ductular epithelial cells are implicated in the secretion of many profibrogenic factors [28]. Sedlaczek et al. [29] found that these cells are the main source of connective tissue growth factor. Moreover, they were found to exhibit increased expression and secretion of neuroendocrine hormones and increased expression of the receptors of these hormones [30]. Targeting the profibrogenic factors secreted by these cells and the neuroendocrine profile could constitute a mechanism for modulating BDP and the progression of liver disease.
Bile duct epithelia that form BDP express P2Y receptors [31]. P2Y receptors are present in many human tissues and are responsible for various biological functions [32]. It has been shown that extracellular nucleotides can stimulate BDP via P2Y receptors [33]. Hence, it is hypothesized that blockade of the P2Y receptors can suppress BDP. Moreover, signaling via extracellular nucleotides could be another target for the suppression of BDP.
The postsurgical outcome of BA is not associated with just a single factor despite many studies concentrating on the age at KP. Moreover, there is still a lack of understanding about the relationship between histopathology and clinical outcome [34]. Muthukanagarajan et al. [7] and Gunadi et al. [8] reported poor outcomes in children with advanced hepatic fibrosis, whereas Santos et al. [35] reported a lack of such correlation. Despite these observations, it is believed that suppression of ongoing rapid fibrosis to avoid consequent portal hypertension is still a therapeutic target.
A possible limitation of this study is the comparison between a diagnostic Tru-cut biopsy (first liver biopsy) and an intraoperative wedge biopsy (second liver biopsy). Higher grades of fibrosis might be reported in the wedge biopsy due to the inclusion of the subcapsular area with possibly more amount of fibrosis. Rawlins et al. [12] found a concordance between a Tru-cut needle biopsy and a wedge biopsy in terms of reporting of fibrosis when the wedge biopsy was sampled 2 mm deeper to the capsule. In the present study, this issue was avoided by sampling the wedge biopsy 5 mm deeper to the capsule.
A strength of this study lies in the assessment of changes in fibrosis and correlation of these changes with other histopathological changes within a short time interval, which is different from some of the previous studies [2] that assessed this correlation after a long time interval. Moreover, studying the relationship of fibrosis progression with histopathological changes could be more informative than merely assessing its relationship with static pathological findings. It was apparent that cases with a greater increase in BDP would exhibit a more significant increase in fibrosis.
In conclusion, the present study provided an important insight regarding the orchestrators of fibrosis progression in BA. It demonstrated that fibrosis is rapidly progressive and the driving force for this unique rapid progression is not arbitrary or related to time alone. A younger infant could exhibit a higher grade of fibrosis. Moreover, an infant could exhibit progression from a lower grade of fibrosis to a higher grade within a shorter time interval compared to the progression of fibrosis in other infants within a longer time interval. Increase in BDP was the only independent factor that showed a correlation with fibrosis progression. Assessment of manipulating BDP as an adjuvant therapy for this devastating disease to prevent or to ameliorate the rapid progression of fibrosis could be considered.
Financial supportThis research received funding from the National Liver Institute, Menoufia University, Egypt, without its involvement in the research design, enrolment of participants, data collection and analysis, or the report write-up.
Author's contributionsEl-Araby HA, Saber MA, Radwan NM, Taie DM, Adawy NM, Sira AM involved in the study concept and design; El-Araby HA, Saber MA, Radwan NM, Sira AM involved in recruitment of patients, clinical management and follow up, and contributed to data acquisition; Taie DM performed the histopathological examination; Sira AM performed statistical analysis and designed the figures; El-Araby HA, Saber MA, Radwan NM, Taie DM, Adawy NM, Sira AM performed data interpretation; El-Araby HA, Saber MA, Radwan NM, Adawy NM, Sira AM wrote the manuscript; all the authors reviewed the manuscript and finally approved it for submission. Ahmad M Sira is the corresponding author.
Conflict of interestThe authors declare that they have no competing interests.
We would like to thank the residents and the nursing staff of the Department of Pediatric Hepatology, Gastroenterology, and Nutrition and all physicians and working staff of the Pathology Department for their contribution.