Introduction and Objectives: Assessment of liver biopsy sample adequacy criteria is essential to avoid sampling errors in patients with diffuse liver pathology. Many studies have evaluated these criteria in adults; however, no previous studies have been performed on neonatal liver disorders. We aimed to assess the adequacy criteria of Tru-cut needle liver biopsy samples in infants with neonatal cholestasis (NC). Methods: In a retrospective analysis of infants who underwent liver biopsy for NC within a one-year duration, 58 specimens were recruited. The core lengths after fixation were measured. All samples were acquired with a 16-gauge (G) Tru-cut needle. Serial shortening of these samples was performed to define the smallest core length that gives representative parenchyma that could determine the activity grade and fibrosis stage reported by larger cores. Results: It was found that a 4-mm core length with a complete portal tract (CPT) number of 8±3 could adequately assess the NC activity grade. In addition, a 6-mm core length with a CPT number of 11±3 could adequately estimate NC fibrosis stage. Conclusions: The adequacy criteria of liver tissue samples for the accurate assessment of NC are different from those defined for adult diffuse liver pathology. At least a 4-mm core length with a CPT number of 8±3 and a 6-mm core length with a CPT number of 11±3 acquired by a 16-G Tru-cut needle should be used to assess NC activity grade and fibrosis stage, respectively.
Liver biopsy is a cornerstone in the diagnostic workup of many disorders causing neonatal cholestasis (NC). However, sampling errors with insufficient liver specimens could cause a biased pathological diagnosis even with expert pathologists. Given the neonatal liver's nonspecific pathological response to different etiologies, there are overlaps in various disease pathologies [1]. This makes it challenging to define the specific etiology by pathological evaluation.
Moreover, as an invasive procedure with some risks, a sample with good quality that could lead to the correct diagnosis should be acquired to balance this potential risk. This means that the biopsy should be of sufficient size to view a representative amount of parenchyma and the number of complete portal tracts (CPTs). Even a very small biopsy specimen may be enough to establish a diagnosis when the disease's key lesion is present [2].
Many studies have defined adequate liver biopsy criteria in those with diffuse pathology in adults [3]. However, no previous studies have defined the adequacy criteria of liver biopsy samples in NC. In the present study, we aimed to determine adequate liver biopsy sample characteristics in NC regarding the core length and portal tract numbers.
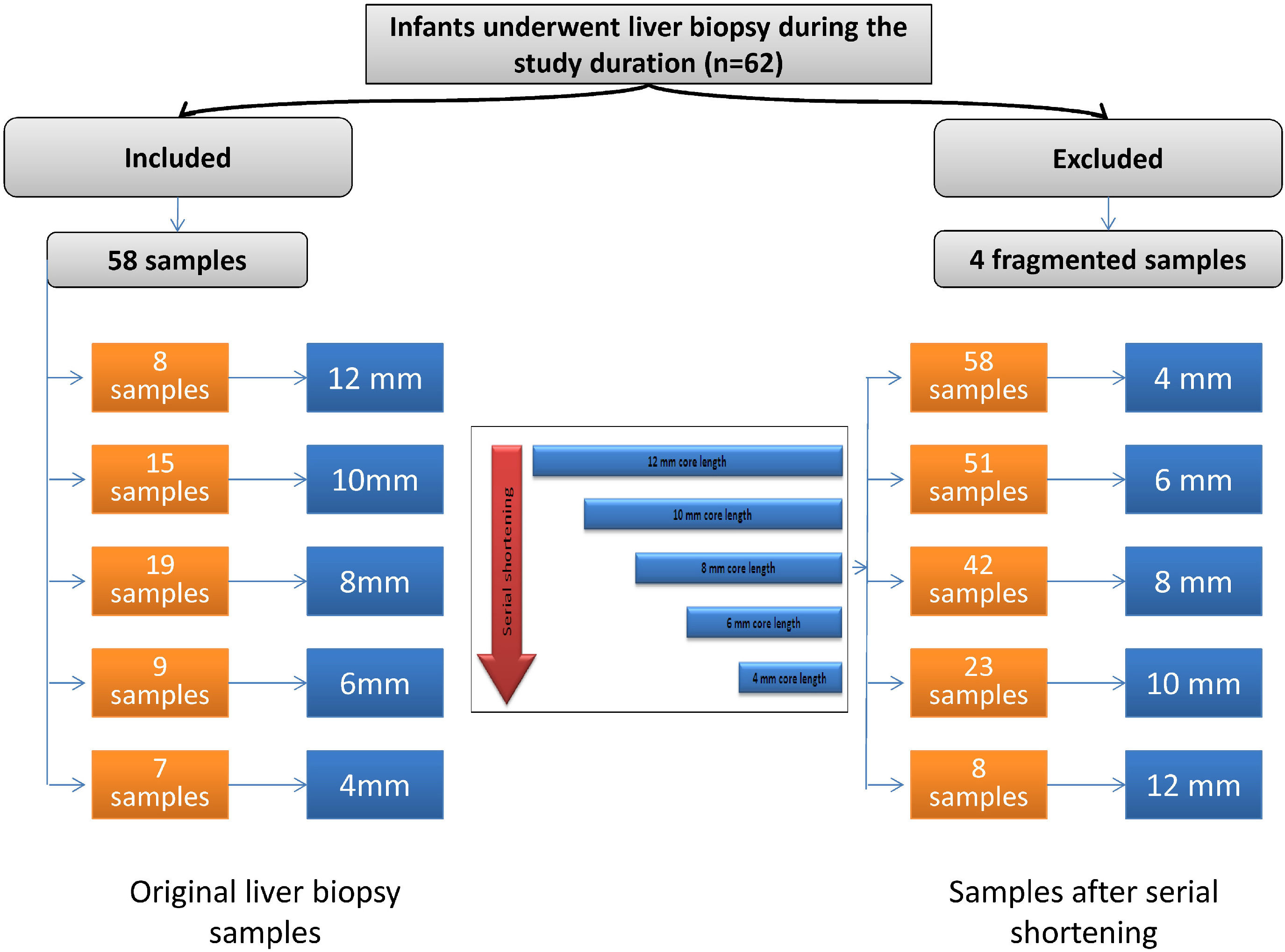
2Materials and methods2.1Study populationThis retrospective study included 58 specimens of liver tissue from infants with NC. All liver specimens of infants with NC admitted to the Department of Pediatric Hepatology, Gastroenterology, and Nutrition who underwent liver biopsy from July 1, 2016, to June 30, 2017, were collected. Within the study duration, 62 infants with NC underwent liver biopsy. Four cases were excluded due to core fragmentation (Fig. 1).
In compliance with the 1975 Declaration of Helsinki and its later amendments, this study's approval was obtained from the Research Ethics Committee of the National Liver Institute. This manuscript does not contain any content that can reveal the patients’ identities; therefore, written informed consent was not obtained from the guardians.
2.2Liver biopsy sampling and processingAll liver biopsy specimens were previously obtained through a Tru-cut needle 16-gauge (G) by ultrasound guidance with one needle pass. After processing of the liver tissue, sections were stained with hematoxylin and eosin, diastase periodic acid-Schiff, orcein, and Masson's trichrome stains for routine histopathological evaluation. The core lengths of all samples after fixation and staining were measured. They ranged from 4 mm to 12 mm. The core lengths were as follows: eight samples, 12 mm; fifteen samples, 10 mm; nineteen samples, 8 mm; nine samples, 6 mm; and seven samples, 4 mm (Fig. 1).
For each core of liver tissue with a length >4 mm, serial shortening by 2 mm was performed until reaching the 4-mm core length. Accordingly, we acquired the following core lengths: 58 samples with a core length of 4 mm, 51 samples with a core length of 6 mm, 42 samples with a core length of 8 mm, 23 samples with a core length of 10 mm, and eight samples with a core length of 12 mm (Fig. 1).
2.3Assessment of adequacy criteriaAn adequate sample is a liver tissue specimen that can express the different pathological criteria without difference from the larger samples. To assess adequacy, we considered the pathological criteria defined by the largest core size as the internal reference standard. Determining the etiological diagnosis was out of the scope of this study in the determination of the adequacy criteria. We relied on defining adequacy to determine the adequate core length and CPT number, while width was that of the 16 G Tru-cut needle.
The current study design of serial shortening of samples to assess the adequacy criteria has been performed before in adults’ studies by Colloredo et al. [4]. In the present work, we compared the pathological scores reported by the smallest available core length (4 mm) to those reported by the next largest core length (6 mm) to define the adequate core length. Then, we compared the 6-mm core length to the 8-mm core length to reach the largest available core length (12 mm). The smallest core length that could define the pathological scores with no significant difference from those reported by the larger core lengths was considered adequate.
The CPT numbers of the defined adequate core length were reported, and the mean values were considered in the definition of the criteria of the adequate liver biopsy core. However, the width was standardized to the width of the liver biopsy cores obtained by the Tru-cut needle 16 G. It was not feasible to obtain different widths for comparison.
2.4Histopathological evaluationFor each liver section, definite pathological parameters were recorded and summed into two items: activity grade (score: 0-15) and fibrosis stage (score: 0-4). The activity grade included the following nine pathological items we reported previously [5]: (1) bile ductular proliferation, (2) bile plugs, (3) portal infiltrate with lymphocytes, (4) neutrophils, and (5) eosinophils, (6) giant cell transformation of hepatocytes, (7) hepatocyte swelling, (8) rosette formation, and (9) extramedullary hematopoiesis. All reported items were given scores; higher scores indicate a greater possibility of biliary atresia rather than nonbiliary atresia cholestasis [6] (Table 1).
Neonatal cholestasis histopathological activity score and fibrosis stage
Liver fibrosis was classified into five stages [7], as reported in Table 1. Two senior pathologists interpreted all histopathological findings. All pathological criteria were assessed three times, one month apart, and median values were reported. The intra- and interobserver kappa values for different pathological parameters were 0.865–0.932 and 0.813–0.842, respectively. For the histopathological findings that showed no concordance between the two pathologists, a shared reassessment was performed.
2.5Statistical analysisQuantitative data are expressed as the mean ± standard deviation. Kendall's coefficient of concordance was used to assess agreement among different core lengths. It was done on two-by-two comparisons and didn't compare all biopsy lengths at the same time. This test results in a number that varies from 0 (complete disagreement) to 1 (complete agreement) [8]. We also performed one-way analysis of variance (ANOVA) to analyze differences in the means of the dependent variable (score) when stratified by the levels of the independent variable (biopsy length). The kappa coefficient was calculated to measure the intra- and interobserver agreements. A P-value of < 0.05 was used to indicate statistically significant results. SPSS 21.0 (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analysis.Results
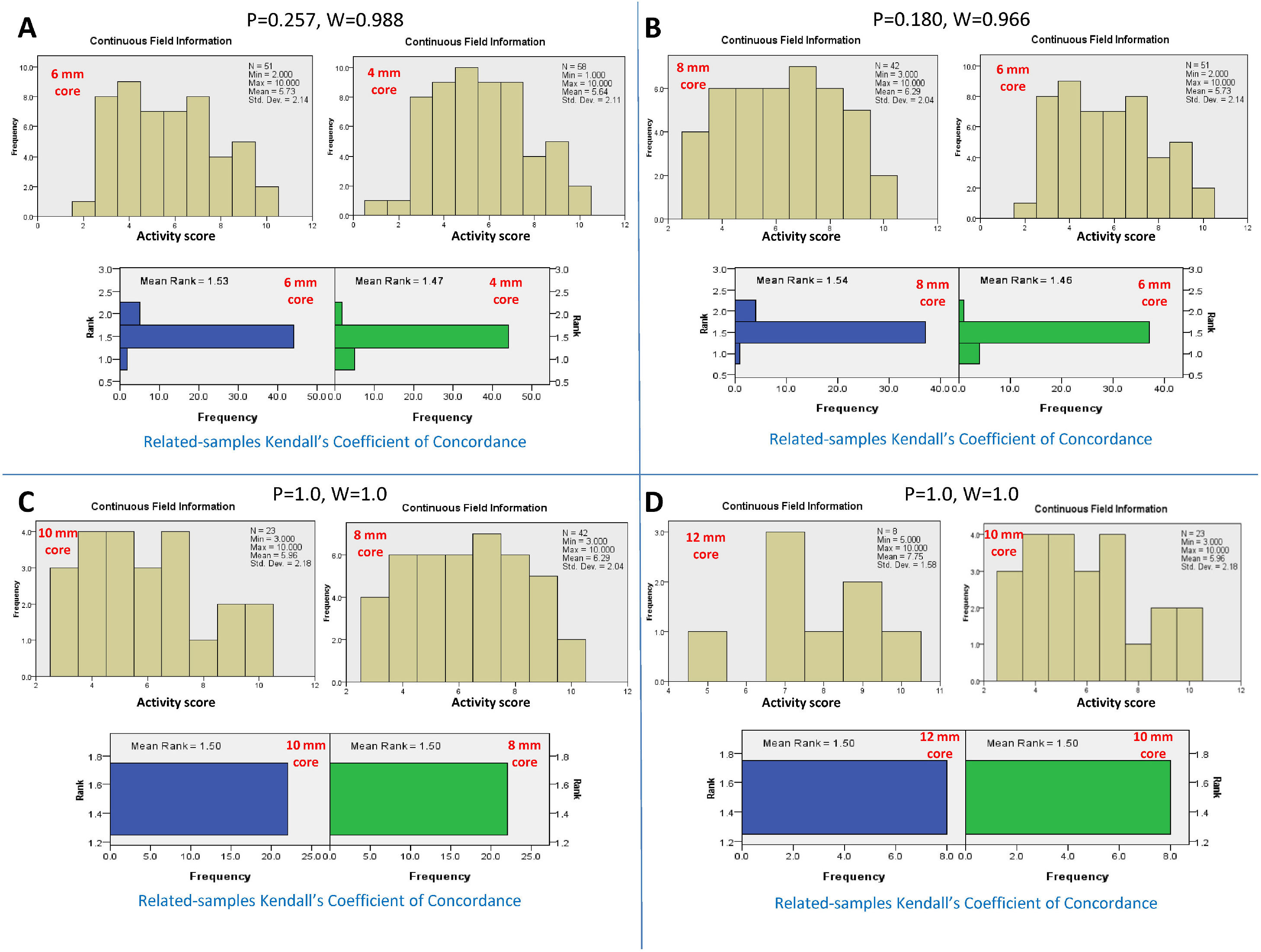
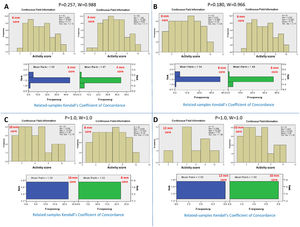
3Adequate core length for the NC activity3.1Adequacy criteria for the activity gradeBy serial comparison of the activity score reported by the 4-mm core length to that reported by the larger core length and so on, it was found that the 4-mm core length was concordant with the 6-mm core length, P = 0.257 and W=0.988. The 6-mm core length was concordant with the 8 mm core length, P = 0.180 and W=0.966. The 8-mm core length was concordant with the 10-mm core length, P = 1.0 and W=1.0. Finally, the 10-mm core length was concordant with the 12-mm core length, P = 1.0 and W=1.0. Therefore, the adequate core length for reporting the total activity grade of NC was 4 mm (Fig. 2).
Kendall's coefficient of concordance for the assessment of the adequate core length for the activity grade. A) Comparison between the activity reported by the core lengths of 4 and 6 mm, P=0.257. The upper diagram represents the activity scores reported for every core length. The lower diagram represents the ranked means and frequencies for every core length. B) Comparison between core lengths of 6 and 8 mm, P=0.180; C) Comparison between core lengths of 8 and 10 mm, P=1.0; D) Comparison between core lengths of 10 and 12 mm, P=1.0.
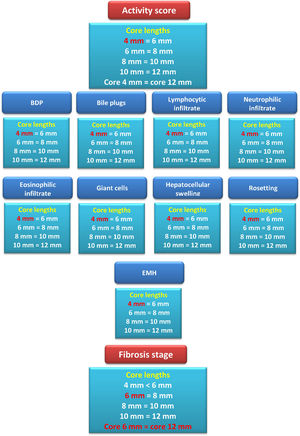
By serial comparisons of different core lengths for every individual histopathological parameter of NC activity, it was found that the 4-mm core length showed concordance with the larger core lengths (P > 0.05 and W=0.966-1.0, for all). Therefore, the adequate core length for the individual parameters of NC activity was 4 mm. Fig. 3 summarizes the results of the comparison of different core lengths for every histopathological parameter.
Summary diagram for comparing different core lengths for every pathological item assessed together with the activity grade and fibrosis stage. For example, the box summarizing the fibrosis stage assessment results showed that the 4-mm core length reported a fibrosis stage lower than that reported by the 6-mm core length. However, the fibrosis stages reported by the core lengths of 6, 8, 10, and 12 mm showed no significant differences among them. Therefore, the adequate core length for reporting the fibrosis stage was 6 mm.
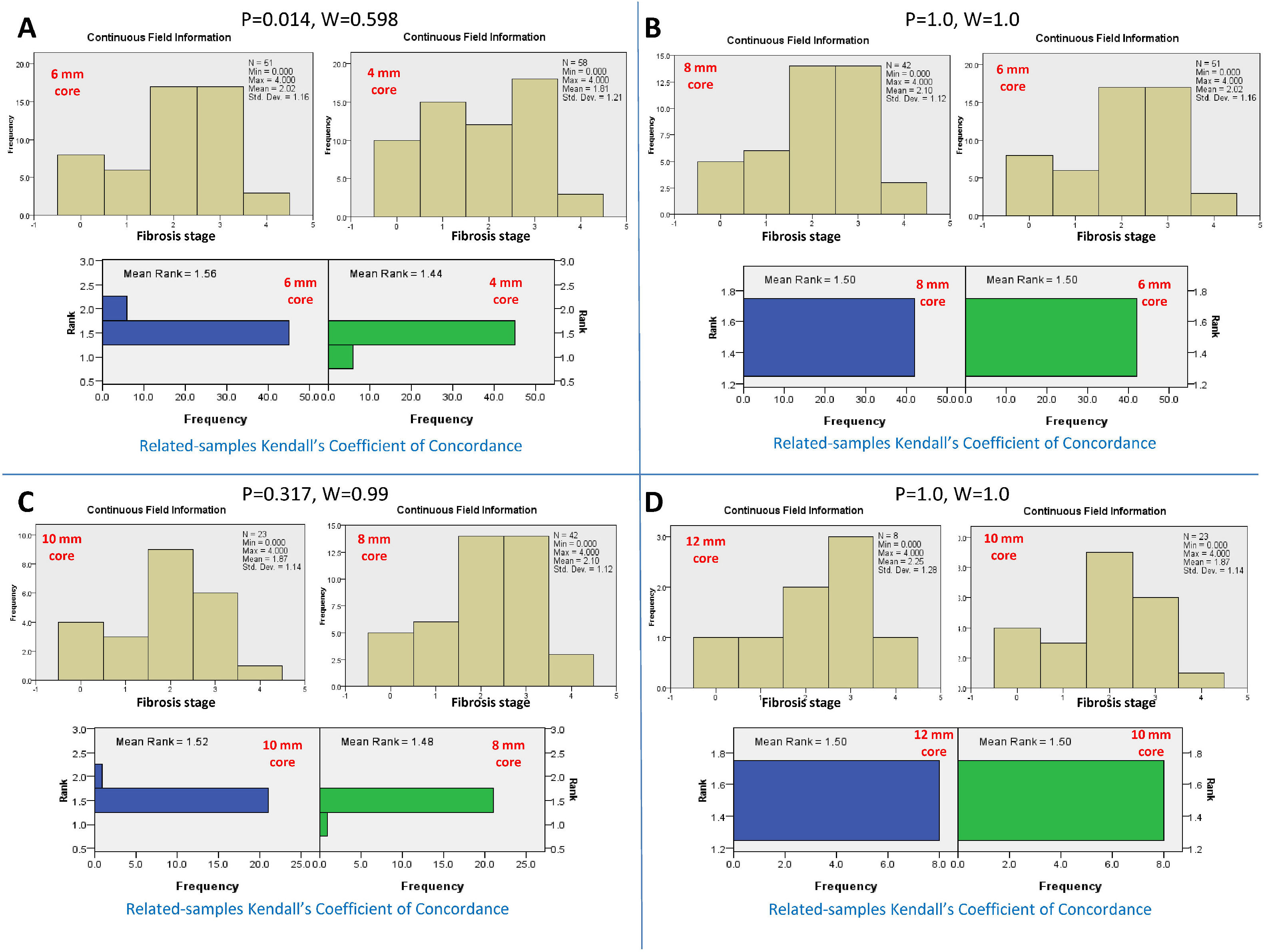
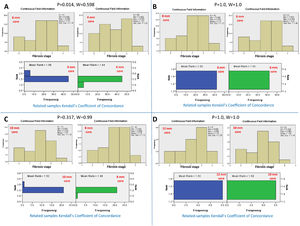
Conversely, serial comparison of core lengths to define the adequate length for fibrosis assessment showed no concordance between the 4-mm core length and the 6-mm core length (P=0.014 and W=0.598). In contrast, there was concordance between serial core lengths of 6, 8, 10, and 12 mm (P>0.05 and W=0.99-1.0, for all). Therefore, the fibrosis stage assessment's adequate core length was 6 mm (Fig.s 3 and 4).
Kendall's coefficient of concordance for the assessment of the adequate core length for the fibrosis stage. A) Comparison between the fibrosis stages reported by core lengths of 4 and 6 mm, P=0.014. The upper diagram represents the fibrosis stage reported for every core length. The lower diagram represents the ranked mean and frequency for every core length. B) Comparison between core lengths of 6 and 8 mm, P=1.0. C) Comparison between core lengths of 8 and 10 mm, P=0.317. D) Comparison between the core lengths of 10 and 12 mm, P=1.0.
The 4-mm core lengths were found to have a CPT number of 8 ± 3. Therefore, the adequate CPT number for reporting activity grade ranged from 5 to 11 per core.
The 6-mm core lengths were found to have a CPT number of 11 ± 3. Therefore, the adequate CPT numbers for reporting the fibrosis stage ranged from 8 to 14 per core.
6ANOVA resultsANOVA of all examined histopathological parameters revealed comparable results to those of Kendall's coefficient of concordance. Moreover, Kendall's coefficient test had the benefit of considering the related-samples concordance and presenting the continuous field information, as shown in Figs. 2 and 4. P-values derived from the ANOVA of the studied parameters were as follows: activity grade=0.17, bile ductular proliferation=0.560, bile plugs=0.810, lymphocyte infiltrate=0.910, neutrophil infiltrate=0.894, eosinophil infiltrate=0.913, giant cells=0.543, hepatocyte swelling=0.506, rosette formation=0.860, extramedullary hematopoiesis=0.992, and fibrosis stage=0.685.
7DiscussionDespite progress in investigational tools and innovative imaging techniques, liver biopsy remains vital in diagnosing many pediatric liver disorders. An adequate sample should be obtained to avoid misdiagnosis as a consequence of sampling errors. The American Association for the Study of Liver Diseases recommended the importance of a sufficient sample size to correctly diagnose diffuse liver disorders [2]. Many studies in adults concluded that the smaller the liver sample was, the less accurate the assessment of both the grade of activity and the stage of fibrosis of diffuse liver disorders [4],[9].
Optimal biopsy length is the subject of debate because an accurate diagnosis of some diseases can be made with short samples. Studies in patients with viral hepatitis have shown that grading and staging accuracy is reduced in biopsies less than 2.0 cm in length [9].
To the best of our knowledge, no studies have evaluated Tru-cut needle liver biopsy adequacy criteria in infants with liver diseases. Data from the adult literature recommend using a 16 G needle and a biopsy length of 2 to 3 cm to ensure adequate biopsy. These defined adequacy criteria allow for more straightforward pathological evaluations with more significant numbers of CPTs visualized than the 18 G needle [2].
The present study revealed that a 4-mm core length is an adequate core length for NC activity grade assessment. It has been shown that this core length gives the same histopathological scores for activity grade as those reported by the larger core lengths of 6, 8, 10, and 12 mm (the highest core length). Moreover, the adequate CPT number for assessing the NC activity grade was found to be 8±3.
In the liver fibrosis assessment, to determine the size of an adequate liver biopsy, the variability of the liver fibrosis distribution within the liver for the particular disease must be known, which is likely to change with the disease stage. Adequate biopsy samples need to be considered in light of the degree to which small inadequate biopsies tend to underestimate both disease grade and stage [10].
The present study revealed that the 6-mm core length is the adequate core length for reporting the fibrosis stage. This core length showed concordance with the fibrosis stages reported by the larger core lengths of 8, 10, and 12 mm (the highest core length). Additionally, the adequate CPT number for assessing the NC fibrosis stage was found to be 11 ±3.
The larger core length required for assessing the NC fibrosis stage than that needed for evaluating the NC activity grade follows the previous recommendation that adequate liver biopsy would be larger when there is a need to assess the stage of fibrosis. Moreover, if cirrhosis is suspected, a cutting rather than a suction needle is recommended [2].
Colloredo et al. [4] evaluated the effects of core length and width on the grading and staging of diffuse liver pathology. The methodology consisted of progressively reducing the original samples’ length and width, all at least 2.5-3 cm long. Their study provided evidence that both the length and the width of the biopsy core affect the grading and staging and that examining shorter and thinner samples leads to underestimating disease severity. The result was that a 20-mm length is the ‘minimum’ recommended size of a liver biopsy containing a number of CPTs ≥11 for the histological assessment of activity and fibrosis in chronic viral hepatitis in adults.
The American Association for the Study of Liver Diseases guidelines recommended that a biopsy of at least 2-3 cm in length, 11 CPT, and 16 G in caliber is recommended for the accurate diagnosis, grading, and staging of fibrosis in diffuse liver disorders in adults [2].
The use of liver biopsy guidelines in clinical practice from the Royal College of Pathology, British Society of Gastroenterology, and the Royal College of Radiologists recommended that a 16 G needle be used for the percutaneous approach. However, an 18 G needle should be used for percutaneous biopsy of a solid lesion, and the length of the sample should be ≥20 mm [3].
The Royal College of Pathologists published its first guidelines on liver biopsy in 2008 and updated its guidelines in 2014 to suggest minimum adequacy requirements for samples to be at least 10 mm long and to contain six portal tracts [11]. These guidelines have now been revised to ensure that all recommendations are up to date. In the third set of guidelines published in 2020 state that if the liver biopsy is adequate, it should be at least 20 mm in length obtained by a 16 G needle and contain at least ten CPTs. A biopsy of less than 10 mm is of limited diagnostic value, and a second pass should be considered. If the biopsy core is between 10 and 20 mm in length, the diagnosis may be compromised. A second pass should be considered, especially if the biopsy's main indications are staging fibrosis [12].
Although adult studies have recommended a biopsy length of 20 mm, the adequacy of a smaller sample size was apparent in the present study. This difference could be attributed to some technical factors. The ratio of the obtained sample to the relatively smaller infant liver approximates the ratio used in adults. Moreover, this difference could be due to the use of core-aspiration needles, such as Menghini needles, in some adult studies[3],[4], which are less traumatizing and can allow for the acquisition of a longer core than the Tru-cut needle used in our study. On the other hand, Menghini needles were avoided, in our study, because the samples obtained tend to be more fragmented. Some adult studies used more than one pass to acquire the target core length[2],[13]. We avoided multiple needle passes in the present study to decrease the biopsy-related complications associated with multiple passes [2].
Understanding the differences between the liver pathology in neonates and infants from those in adults explains the differences in liver specimen adequacy criteria between pediatric patients and adults. Application of the adequacy criteria reported in adults would miss the diagnosis in many neonates and infants who could be accurately diagnosed with smaller specimens than those defined from adult studies.
A limitation of this study is the retrospective nature, which could not permit a comparison between different needle sizes: 16 G vs. 18 G. Meanwhile, it was challenging to acquire different widths from the studied cores in the present study. However, in our center, we routinely use Tru-cut needles 16 G because our former 18 G needles yielded fragmented tissue samples with fewer CPT numbers. Palmer et al. [13] reported that specimen adequacy was not significantly affected by the biopsy gauge of 16 G vs. 18 G. However, other studies reported that 16 G needles yielded better quality samples with a higher CPT number than 18 G needles [13, 14].
In conclusion, to the best of our knowledge, the present work is the first to assess liver biopsy sample adequacy criteria for the accurate assessment of neonatal liver disorders, namely, NC. A liver specimen acquired with a 16 G Tru-cut needle with a 4-mm length and a CPT of 5-11 is accurate for activity grading. However, to stage fibrosis for NC, a 16 G Tru-cut needle sample should be at least 6 mm in length with CPT numbers of 8-14.
Financial supportThis study was funded by the National Liver Institute, Menoufia University, Egypt. The funders had no particular role in the study design, recruitment of individuals, data analysis, or writing of the report.
Author contributionsBehairy BE, Allam AA, Hegazy SG, Taie DM, and Sira AM were involved in the study concept and design; Behairy BE, Allam AA, Hegazy SG, and Sira AM were involved in the recruitment of patients, clinical management and follow-up and data acquisition; Taie DM performed the histopathological examination; Sira A performed the statistical analysis and designed the figures; Behairy BE, Allam AA, and Sira AM performed data interpretation; Behairy BE, Allam AA, Taie DM, and Sira AM wrote the manuscript; all the authors reviewed the manuscript and ultimately approved it for submission.