Background/aims. Hepatocellular carcinoma is one of the most commonly diagnosed malignant tumors in the world, and it typically has a poor prognosis. Extensive studies have examined the effects of non-steroidal anti-inflammatory drugs selective to COX–2 on the chemoprevention of various tumors. The objective of this study is to observe the effect of celecoxib on the development of liver tumors in rats.
Material and methods. Hepatocellular carcinoma was induced in a group of 75 rats with the carcinogen diethylnitrosamine. The animals were divided into 5 groups. Three groups received various doses of celecoxib, one group received indomethacin, and a control group received no non-steroidal selective anti-inflammatory drugs.
Results. The experimental model was considered to be successful because 78% of the rats in the control group developed liver tumors. The number of neoplastic lesions was similar among the celecoxib, indomethacin and control groups, although the nodule diameter of the lesions was smaller in the celecoxib group. Better results were observed in animals that received celecoxib at doses of 6 and 9 mg/kg/ day; 4 rats in these groups did not show any neoplastic histological lesions, and a greater proportion of the nodules in the other animals in these groups were benign than in the groups that did not use celecoxib.
Conclusions. These results suggest that celecoxib may play a role in modifying the natural history of hepatocellular carcinoma development.
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor. Estimates have indicated that liver cancer remains the fifth most common malignancy worldwide. The number of new cases has been estimated to be 600,000 per year. HCC is a global health problem and is the third most frequent cause of death by cancer worldwide.1,2
The incidence of HCC is increasing in Europe and the United States, and this disease is currently the leading cause of death among cirrhotic patients.1,3,4 In 2009, a Brazilian survey showed that the hepatitis C virus was the most common cause of liver disease in patients with HCC.5 In addition to its increasing incidence, the prognosis of HCC is extremely poor; the 5-year rate of survival after diagnosis is less than 10%.3,4,6
Many treatment options are available for HCC, including surgical resection, orthotopic liver transplantation, percutaneous ethanol injection, radiofrequency thermoablation, chemoembolization1,7 and, more recently, the use of molecular targeted therapy (sorafenib).8,9 However, there are some obstacles to the implementation of these treatments. Loss of hepatic function is a particularly important obstacle because HCC patients often also have cirrhosis. In addition to the loss of hepatic function, the possibility of multicentric neoplastic disease substantially reduces the response to these proposed treatments. Most patients are diagnosed at advanced stages, so an urgent need exists for new systemic therapies.10
Some systemic drugs may act as prophylactics against the appearance of lesions with malignant potential in patients with chronic liver disease. In addition, systemic therapies could act as co-adjuvants with existing treatments.11
Cycloxygenase-2 (COX-2), a key enzyme in arachidonic acid metabolism, is overexpressed in many types of malignant tumors. The overexpression of COX-2 is associated with carcinogenesis in colorectal, prostate, and breast cancers and in hepatocellular carcinoma. Inflammatory mediators, such as prostaglandins (PGs), are implicated in hepatic carcinogenesis, and increased COX-2 expression has been observed in human and animal HCCs. The frequency of aberrant COX-2 expression increases gradually in the progression from chronic hepatitis to cirrhosis to dysplasia. COX-2 expression is generally higher in well-differentiated HCCs than in lessdifferentiated HCCs or histologically normal livers, suggesting that COX-2 may be involved in the early stages of hepatocarcinogenesis.12–17
Several studies have shown that COX-2-derived prostaglandins promote hepatocarcinogenesis through the regulation of several key events, including tumor cell invasion, proliferation, apoptosis, and angiogenesis.15,16
The overexpression of COX-2 or treatment with exogenous PGE2 increases human HCC cell growth and invasiveness.18,19 COX-2 is not constitutively expressed but is rapidly induced by both inflammatory and mitogenic stimuli, resulting in increased PG synthesis in neoplastic and inflamed tissues.17
Experimental studies in animal models of liver cancer have shown that non-steroidal anti-inflammatory drugs (NSAIDs) exert chemopreventive and therapeutic effects.16 In hepatocarcinogenesis, the use of chemoprophylaxis with non-selective NSAIDs has already been tested, and a reduction in the number and size of preneoplastic induced lesions has been observed in rats submitted to a choline-deficient diet.18,20
Kern, et al.21 have identified exaggerated expression of COX-1 and 2 in hepatic tumor cell lines. They demonstrated a reduction of up to 80% in the number of tumor cells upon treatment with selective and non-selective COX-2 inhibitors. The authors concluded that the selective inhibition of COX-2 could have important therapeutic and preventive potential in human hepatocarcinogenesis by inhibiting hepatic tumor cell growth through the induction of apoptosis and inhibition of cell proliferation.
The treatment of HCC cell cultures with several COX-2 inhibitors induces marked apoptosis, as reflected by morphological evidence of cell death.15 The selective COX-2 inhibitor NS-398 inhibits the growth of HepG2 and Huh7 cells by inducing cell cycle arrest22 and apoptosis.23 In addition, inhibitors of COX-2 can significantly repress the expression of angiogenesis factors.16,24 Evidence suggests that the COX-2 inhibitor reduces the production of VEGF, a potent stimulator of angiogenesis, by HCC cells.15
Celecoxib belongs to the new generation of NSAIDs that selectively inhibit COX-2 activity without the inhibition of COX-1 and thus lack the side effects associated with traditional NSAIDs. Celecoxib inhibits the progression of colon tumors in human and animal models and inhibits the in vivo growth of several other tumor cell types. The observation that celecoxib induces tumor cell apoptosis in cells that lack the COX-2 enzyme also suggests the involvement of a COX-2-independent mechanism in celecoxib-induced tumor cell apoptosis.14,18
COX-2 is believed to be a promising target for antitumor treatment because a wide range of tumor-promoting signal pathways can theoretically be targeted by the selective COX-2 inhibitors. Considering the lack of studies in the literature and the possibility of a new field of HCC therapy, we experimentally evaluated the role of selective COX-2 NSAIDs in the development of liver tumors and determined these agents to be of great importance.
Material And MethodsThis study examined 75 male rats (Rattus norvegicus), the number of animals per group was calculated from data of literature.25,26 All animals were 45 days old, heterozygous and from the Wistar lineage. The animals were obtained from the animal breeding center of Fundação Universidade Federal de Ciencias da Saúde de Porto Alegre (UFCSPA). All studies were performed in accordance with the Guiding Principles for Research Involving Animals at Hospital de Clínicas de Porto Alegre HCPA.27
The rats were maintained in groups of 3 or 4 in polypropylene cages in a room with a controlled temperature of 22 °C (± 2 °C range) and a light/dark cycle of approximately 12 h each (light cycle: 7 am to 7 pm). The animals received standardized meals (Nuvilab CR1 ration by Nuvital Nutrientes Ltda.) and water through glass bottles ad libitum.
The hepatocellular carcinoma induction followed an experimental model similar to that described by Johnson, et al.28 The rats received a solution of 50 mg/liter (prepared in water) diethylnitrosamine (DEN) (Sigma-Aldrich Company, USA) through glass bottles daily for a period of 74 consecutive days.
The 75 rats were randomly divided into 5 groups of 15 animals each and received the following treatments:
- •
Group 1 (DEN). Received the DEN solution (50 mg/L in water) for 74 days and the control solution (NaCl 0.9%, 1 mL/kg/day through gastric probing) for 129 days.
- •
Group 2 (indomethacin). Received the DEN solution (50 mg/L in water) for 74 days and the indomethacin solution (Indocid, 50 mg, Prodome, Brazil) (2 mg/kg/day through gastric probing) for 129 days.
- •
Group 3 (COX 3 mg). Received the DEN solution (50 mg/L in water) for 74 days and the celecoxib solution (Celebra, 200 mg, Pharmacia Brasil, Brazil) (3 mg/kg/day through gastric probing) for 129 days.
- •
Group 4 (COX 6 mg). Received the DEN solution (50 mg/L in water) for 74 days and the celecoxib solution (6 mg/kg/day through gastric probing) for 129 days.
- •
Group 5 (COX 9 mg). Received the DEN solution (50 mg/L in water) for 74 days and the celecoxib solution (9 mg/kg/day through gastric probing) for 129 days.
Previous studies have demonstrated the effect of celecoxib in control animals;29,30 therefore this experimental group was not used.
The bottles with DEN solution were weighed daily to determine the solution intake per cage of animals. The remaining solution in the bottles was discarded in chemical waste containers, and the bottles were refilled with new DEN solution.
The animals were weighed every two weeks throughout the experimental period. After each weighing procedure, the quantities of drugs were adjusted according to the animal’s weight.
One day after the final drug treatment (day 130), the animals were decapitated and submitted to a laparotomy. The liver, fully resected, was weighted on an analytical scale (Marte AS 500 C/Brazil). After weighing, a fragment of each liver was immediately frozen in liquid nitrogen to assay lipid peroxidation. As a control group, 15 rats without any treatment were also examined.
The oxidative stress study used the thiobarbituric acid reactive substances (TBARS) method. The amount of aldehydic products generated by lipid peroxidation was quantified by the TBA reaction using 3 mg of protein/sample. The spectrophotometric absorbance of the supernatant was determined at 535 nm. The results were expressed as nmol of malondialdehyde (MD) per milligrams of protein.31 The protein concentrations in homogenates were measured using the Lowry method32 with bovine serum albumin as the standard. Both measurements were performed in a Perkin Elmer Lambda 40 spectrophotometer.
For the histological analysis, the samples were placed in Bouin fixative and embedded in paraffin twice. Using a microtome, the paraffin blocks were cut into 3-μm serial sections. For staining, the slides were immersed in hematoxylin-eosin. For the dehydration phase, the slides were placed in a series of three containers with absolute alcohol and two containers with xylol. Analysis of the slides was performed using light microscopy (Nikon Labophot) at 100x. The analysis was performed by 2 pathologists who were unaware of the study details.
For the macroscopic study, all solid nodules of 1 mm or larger present on the surface of each liver were numbered. Serial cuts of the entire organ were then performed, with each cut presenting an approximate thickness of 2 to 3 mm. The nodules were then re-counted, and the number of surface nodules was added to those inside the organ. The selection of the minimum nodule size to be observed and the liver cut thickness were similar to those of other experimental studies, such as that conducted by Simile, et al.33
After this initial count, three nodules were removed to identify the histological type.
For the microscopic evaluation, the central slice of the medium lobe was kept intact because it is the largest straight surface area of the organ. This slice was selected as the standard material for the microscopic nodule count.
The criteria adopted for the diagnoses of benign or malignant hepatic lesions followed the guidelines described by Stewart, et al.34
The quantitative data are described as the mean values and standard deviations. In situations of asymmetry, logarithmic transformation was utilized. The qualitative data are described as frequency and percentage.
Analysis of variance (ANOVA) was used as a classification criteria for the comparison of quantitative data between the studied groups and for the oxidative stress index analysis. Additionally, in situations in which the plausibility of a linear effect was identified, a simple linear regression was used to describe the effect of anti-inflammatory drugs on the considered outcomes. In the comparison of qualitative variables, the chi-squared test and Fisher’s exact test were used. Differences were considered significant at 0.05.35
The data were processed and analyzed using the SPSS (Statistical Package for the Social Sciences) program.
ResultsSuccessful results were achieved using the hepatocarcinogenesis experimental model. Malignant nodules were found in 78% of the rats (11 out of 14) in the control group.
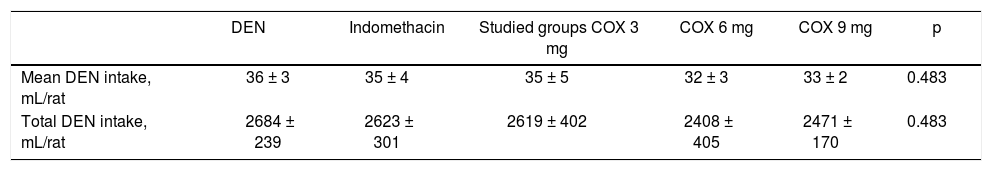
Table 1 shows the evaluation of DEN solution intake per rat in milliliters. Both daily intake and total intake of DEN were analyzed in the first 75 days of the study (period of tumor induction); the results indicated similar intake in all 5 groups of rats (p = 0.483).
DEN solution intake per rat.
| DEN | Indomethacin | Studied groups COX 3 mg | COX 6 mg | COX 9 mg | p | |
|---|---|---|---|---|---|---|
| Mean DEN intake, mL/rat | 36 ± 3 | 35 ± 4 | 35 ± 5 | 32 ± 3 | 33 ± 2 | 0.483 |
| Total DEN intake, mL/rat | 2684 ± 239 | 2623 ± 301 | 2619 ± 402 | 2408 ± 405 | 2471 ± 170 | 0.483 |
Data are presented as the mean values ± standard deviations. DEN: diethylnitrosamine.
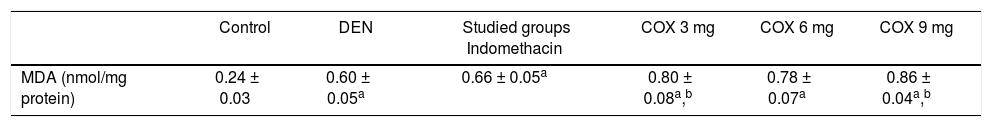
Rats treated with diethylnitrosamine presented higher levels of lipid peroxidation as determined by the malondialdehyde concentration in the liver tissue (control: 0.24 ± 0.03 nmol/mg protein and DEN: 0.60 ± 0.05 nmol/mg protein, p < 0.05). Regarding NSAID treatment groups, lipid peroxidation remained high in all groups, but 3 mg/kg celecoxib (0.8 ± 0.08 nmol/mg protein) and 9 mg/kg celecoxib (0.86 ± 0.04 nmol/mg protein) presented the highest levels compared to the other groups (6 mg/kg Celecoxib: 0.78 ± 0.07 nmol/mg protein and Indomethacin: 0.66 ± 0.05 nmol/mg protein) (p < 0.05). These data are illustrated in table 2.
MDA levels in liver.
| Control | DEN | Studied groups Indomethacin | COX 3 mg | COX 6 mg | COX 9 mg | |
|---|---|---|---|---|---|---|
| MDA (nmol/mg protein) | 0.24 ± 0.03 | 0.60 ± 0.05a | 0.66 ± 0.05a | 0.80 ± 0.08a,b | 0.78 ± 0.07a | 0.86 ± 0.04a,b |
Data are presented as the mean values ± standard deviations. MDA: malondialdehyde. DEN: diethylnitrosamine.
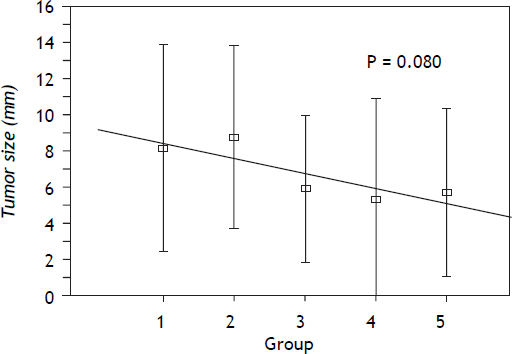
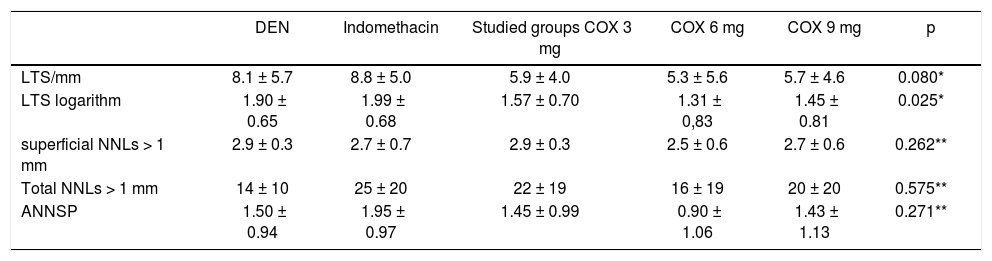
The largest tumor size (LTS) in millimeters and the number of nodules per liver (NNL) found in each rat were macroscopically evaluated (Table 3).
Evaluation of the largest tumor size and the number of nodules per liver.
| DEN | Indomethacin | Studied groups COX 3 mg | COX 6 mg | COX 9 mg | p | |
|---|---|---|---|---|---|---|
| LTS/mm | 8.1 ± 5.7 | 8.8 ± 5.0 | 5.9 ± 4.0 | 5.3 ± 5.6 | 5.7 ± 4.6 | 0.080* |
| LTS logarithm | 1.90 ± 0.65 | 1.99 ± 0.68 | 1.57 ± 0.70 | 1.31 ± 0,83 | 1.45 ± 0.81 | 0.025* |
| superficial NNLs > 1 mm | 2.9 ± 0.3 | 2.7 ± 0.7 | 2.9 ± 0.3 | 2.5 ± 0.6 | 2.7 ± 0.6 | 0.262** |
| Total NNLs > 1 mm | 14 ± 10 | 25 ± 20 | 22 ± 19 | 16 ± 19 | 20 ± 20 | 0.575** |
| ANNSP | 1.50 ± 0.94 | 1.95 ± 0.97 | 1.45 ± 0.99 | 0.90 ± 1.06 | 1.43 ± 1.13 | 0.271** |
Data are presented as the mean values ± standard deviations.
A positive correlation was observed between treatment with a selective COX-2 NSAID and a smaller size of the nodules compared to treatment with indomethacin or NaCl. This tendency was confirmed with a simple linear regression with a value of p = 0.025 and with data in logarithmic format. These data are shown in figure 1.
In addition, information regarding the number of nodules in each organ was obtained by macroscopically evaluating each animal’s liver. For this reason, all nodules of 1 mm or larger on either the liver surface or within the tissue were counted. No differences were detected between the five studied groups in either the surface nodule sum or the total nodule sum of each liver. P values of 0.262 and 0.575, respectively, were obtained. These data are illustrated in table 3.
The number of lesions and their histological type were also observed by microscopic evaluation of the livers. Table 3 shows the results regarding the number of lesions, represented as the average number of nodules on standard plates (ANNSP). No statistically significant differences were observed among the 5 groups (p = 0.271).
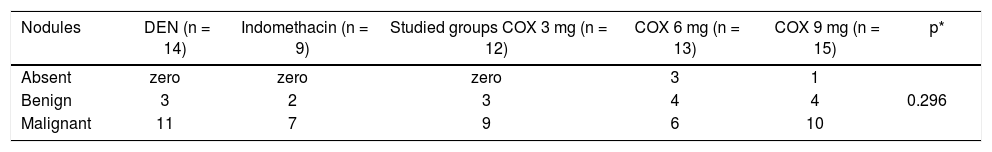
Histological observations indicated that the groups of rats that received celecoxib presented a higher number of benign nodules or no nodules at all compared to the indomethacin group or the control group. The 4 animals that did not present any nodules, either macroscopically or microscopically, belonged to the celecoxib groups. Tables 4 and 5 illustrate these data. However, these results were not statistically significant.
Histological type of nodules analyzed microscopically.
| Nodules | DEN (n = 14) | Indomethacin (n = 9) | Studied groups COX 3 mg (n = 12) | COX 6 mg (n = 13) | COX 9 mg (n = 15) | p* |
|---|---|---|---|---|---|---|
| Absent | zero | zero | zero | 3 | 1 | |
| Benign | 3 | 2 | 3 | 4 | 4 | 0.296 |
| Malignant | 11 | 7 | 9 | 6 | 10 |
The importance of the elevated expression of COX-2 in liver tumors has not been well explained in medical literature. Therefore, studies of the antitumoral activity of selective COX-2 NSAIDs in this neoplasia are being performed in animals or in vitro.
In 1993, Tanaka, et al.36 investigated the possible effects of indomethacin and pyroxicam as treatments against chemically induced hepatocellular carcinoma in rats. They found inhibition of hepatocarcinogenesis in the groups of animals in which treatment with NSAIDs was concomitant with the use of the carcinogen. The authors concluded that a possible correlation exists between the altered metabolism of arachidonic acid and the initial phase of induced hepatic carcinogenesis. Isolated reports also exist of human HCC response to treatment with selective COX–2 inhibitors such as celecoxib.20
Koga, et al.12 investigated COX-2 enzyme expression in hepatic tumors by evaluating resected surgical specimens of 44 patients with HCC using immunohistochemical and immunoblotting techniques. The authors used 7 histologically normal livers as control resected specimens. A frequently higher expression of COX-2 was found in differentiated HCCs and decreased or absent expression of COX-2 was observed in differentiated HCCs and normal livers. The authors concluded the study by suggesting that COX-2 participates in the initial stages of hepatocarcinogenesis and that it may correlate with the HCC differentiation status. Years later, Koga14 emphasized again the possible role of COX-2 in the early phases of hepatocarcinogenesis, drawing the attention to the correlation between the increased presence of the enzyme in hepatic tissue and a shorter time free of disease in patients with HCC.
Some studies have indicated the importance of the elevated expression of COX-2 in the early phases of hepatocarcinogenesis, emphasizing that the enzyme is increased in livers with cirrhosis and in highly differentiated HCCs but not in undifferentiated neoplasias. Elevated expression of COX-2 in nonneoplastic hepatic tissue would be associated with a higher recurrence of HCC after resection of a malignant nodule.37 A strong association was found between the degree of differentiation of HCC cell lineages and COX-2 expression. COX-2 may determine the degree of cell differentiation in HCC, and COX-2 inhibition may suppress cell growth via the induction of apoptosis and inhibition of angiogenesis.11,38
Recent studies have reported that celecoxib results in increased levels of activated caspase-3 and caspase-9 but decreased Bcl-2 expression in vitro. Thus, celecoxib promotes apoptosis of hepatocellular carcinoma cells by modulating the activation of caspase-3 and caspase-9, which seems to be independent of the level of COX-2 expression.13
The inhibition of serine/threonine kinase B (Akt) has been shown to be involved in the potent antitumor effects of celecoxib. Studies in human HCC tissue samples have suggested that the utilization of celecoxib would reduce the phosphorylation of Akt, a key mediator of signal transduction with a central role in carcinogenesis, and would induce apoptotic characteristics at morphological and biochemical levels.18,21,29
Some in vitro studies have pointed to a correlation between expression of the MDR1 phenotype and expression of the COXP-2 enzyme and induced nitric oxide synthase (iNOS). There was a significantly higher expression of COX-2 and iNOS in cell lineages that expressed MDR1. Additionally, with the utilization of COX-2 specific NSAIDs, a reduced expression of messenger RNA for both COX-2 and iNOS was observed. The MDR1 phenotype causes a constitutive expression of COX-2 and iNOS in HCC cells, correlating the phenotype with tumor angiogenesis. PGE2, as well as nitric oxide, may protect the cancer cells and contribute to tumor growth.40 Confirmation of selective COX-2 NSAID action on disease development may mean that patients who are at risk of developing HCC, such as those with cirrhosis, could benefit from the chemopreventive action of these anti-inflammatory drugs.41
Bannasch42 found that the sequence of morphological and molecular alterations to the hepatocytes during experimental chemical hepatocarcinogenesis in rats is similar to that found in human liver tumor development. An experimental hepatocarcinogenesis model in rats is therefore useful to our understanding of hepatic disease in humans.
This study used the experimental hepatocarcinogenesis model described by Johnson, et al.28 and considered the weight-dependent DEN solution concentration and the length of animal exposure. It should be noted that, as opposed to Johnson’s work, the rats in this study were male.
The absence of statistically significant differences in DEN solution intake among the 5 different groups of rats during the experiment allowed the authors to compare the effect of the studied drugs without the influence of a possible disparity in the carcinogen intake among the groups. Such a disparity could affect responses to various NSAIDs.
The DEN group presented a hepatic malignant nodule incidence of 78% on the 130th day of the study (11 out of 14 animals). This rate was higher than that of the original model, which reported the presence of HCC in approximately 61% of the rats in the same period (11 out of 18 tested animals).
Reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide and hydroxyl radical, are known to be important in the pathophysiology of ischemia-reperfusion injury, in the toxicity induced by several xenobiotics, in cardiac and lung toxicities induced by air pollution and in mutagenesis and carcinogenesis.43–45 ROS can be directly involved in oxidative damage to lipids, proteins and DNA.43 The results of this study suggest that lipid peroxidation plays an important role in the induction of this tumor in an animal model. Lipid peroxidation was not modified by NSAIDs treatment, although the level of lipid peroxidation was lower in the celecoxib (6 mg/kg) group.
A macroscopic examination of the animals’ livers revealed a reduced tumor size in animals that received celecoxib. This association occurred with the three distinct dosages of the drug but was most significant in the 6 mg/kg/dose group. Although a smaller tumor size was associated with the use of celecoxib, the total number of nodules per liver, either macroscopically or microscopically, did not change; celecoxib could reduce the tumor size but not its multiplicity.
Fantappiè, et al.40 observed that the action of PGE2 favored tumor growth. Therefore, by using a selective COX-2 NSAID, we would be inhibiting this process. The present study’s finding of smaller-sized tumors confirms this assumption. In animal model of carcinogenesis, celecoxib treatment resulted in both induction of apoptosis and inhibition of proliferation. In contrast, indomethacin was found to inhibit cell proliferation without induction of apoptosis in tumors. The mechanisms underlying the chemopreventive effect of celecoxib may be more related to its ability to induce apoptosis which was not found in indomethacin-treated group.46 This fact could justify the data found in the present study.
When evaluating the nodules for benignity or malignity, even without a statistical difference, favorable results were obtained in the groups that took celecoxib. Eleven animals out of 40 presented benign nodules (approximately 27%), while in the control and indomethacin groups, 5 out of 23 animals presented benign nodules (approximately 21%). Four animals did not present any type of neoplastic lesion, either benign or malignant; all of these animals belonged to the groups that took celecoxib (three in the 6 mg/kg dosage group and one in 9 mg/kg dosage group). There was a reduction of 10% in the formation of nodules in the animals treated with celecoxib (Table 5). It seems that the dose of 6 mg/kg of celecoxib is more effective that the dose of 9 mg/kg, therefore beyond presenting a lesser number of malignant nodules, some animals had not developed any type of nodule. These data go of meet-ing to the lipoperoxidation findings. These results, although promising, were not statistically significant in the tests performed.
Histological evaluation regarding the presence of nodules.
| Celecoxib intake | Rats (n) | Nodules Frequency (%) | p* |
|---|---|---|---|
| Yes | 40 | 36 (90) | 0.287 |
| No | 23 | 23 (100) |
Experimental studies also revealed that celecoxib inhibited the growth of cancers in vitro and in vivo. Treatment with celecoxib significantly inhibited the proliferation of H22 cells in a dose- and time-dependent manner. The dose of celecoxib (50–400 μmol/L) used was higher than that used in anti-inflammatory therapy. In the present study, high doses of celecoxib (200 and 400 μmol/L) significantly inhibited the mRNA and protein expression of COX-2 and decreased PGE2 levels in H22 cells. However, low doses of celecoxib (50 and 100 μmol/L) did not affect the mRNA and protein expression of COX-2 or PGE2 production.47 It was also found that 10 μmol/L celecoxib reduced P-glycoprotein, Bcl-xL, and Bcl-2 expression in MDR-positive hepatocellular carcinoma cells. Thus, the use of low doses of celecoxib is promising approach to improving cancer chemotherapy outcomes. Celecoxib, which directly reduces P-glycoprotein expression, might enhance the susceptibility of tumor cells to apoptosis induced by anticancer drugs.48
The difficulty of handling animals in experiments prevented the utilization of a large number of rats in each group. The absence of statistically significant results may result from the small number of rats employed in the study (15 animals per group) rather than from a lack of celecoxib activity. The carcinogenesis induction model employed in the study should also be considered. A high percentage of tumors were observed in the control group; perhaps if a smaller dose of DEN had been applied, then it would have been possible to observe a more accentuated effect. In other words, the carcinogenic potential of DEN was be very high when compared to the possible protective effect of selective COX-2 NSAID.
As a result of the evidence found in the literature and the findings of this study, we suggest that celecoxib can potentially affect the development of liver tumors. Further studies with a greater sample of animals and a smaller dose of DEN or a higher dose of celecoxib may contribute important information about the action of selective COX-2 NSAIDs in the development of hepatic neoplasias.
Abreviations- •
Akt: serine/threonine kinase B.
- •
ANNSP: average number of nodules on standard plates.
- •
Bcl-2: B-cell lymphoma 2.
- •
Bcl-xL: B-cell lymphoma-extra large.
- •
COX-2: cycloxygenase-2.
- •
DEN: diethylnitrosamine.
- •
HCC:- hepatocellular carcinoma.
- •
iNOS: induced nitric oxide synthase.
- •
LTS: largest tumor size.
- •
MD: malondialdehyde.
- •
MDR1: multidrug resistance protein 1.
- •
n: number of animals.
- •
NaCl: sodiun cloride.
- •
NNL: number of nodules per liver.
- •
NSAIDs: non-steroidal anti-inflammatory drugs.
- •
PGs: prostaglandins.
- •
ROS: reactive oxygen species.
- •
SPSS: Statistical Package for the Social Sciences.
- •
TBA: thiobarbituric acid.
- •
TBARS: thiobarbituric acid reactive substances.
Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) of the Ministério da Educação e Cultura.