Objectives. To assess the efficacy of anti-viral therapy on hepatitis B virus associated glomerulonephritis (HBV-GN).
Design, setting,participants, & measurements. We searched PubMed, Embase and Cochrane Library for prospective controlled trials which assessed the efficacy of anti-viral therapy on HBV-GN in adult or pediatric patients between January, 1970 and October, 2010.
Results were summarized using fixed-effects model because of an absence of heterogeneity among the studies (I2 = 0%). Results. Six trials with a total of 159 patients were included; among them five trials were specified as hepatitis B virus-associated membranous glomerulonephritis (HBV-MN). In adult patients, the incidence of proteinuria remission, not only total remission (complete remission CR + partial remission PR) (2.97 to 109.93, P = 0.002) but also CR (1.18 to 16.11, P = 0.03), significantly increased in the anti-viral treatment. In pediatric patients, only the incidence of total remission (1.77 to 17.75, P = 0.003) was increased significantly; the incidence of CR was not pooled with clinical and statistical heterogeneity (I2 = 81.5%, P = 0.004).Combine the data from adult and pediatric patients with HBV-MN, the same results were found. All the results of proteinuria remission kept with virologic response (VR), including HBeAg conversion (5.68 to 40.04, P < 0.00001) and reduction of HBV-DNA (5.60 to 463.16, P = 0.0005).
Conclusions. Antiviral therapy including IFN and lamivudine is effective on remission of proteinuria, HBeAg clearance, and HBV-DNA reduction.
With the increment of incidence on extrahepatic infection, the effective treatment for renal related hepatitis B-virus (HBV) infection has attracted more attention recently.1-4 Epidemiological studies have shown that chronic carriage of HBV in some individuals (particularly pediatric patients) leads to the development of nephritic syndrome with a strong male predominance, the commonest histological type being membranous nephropathy (MN).5,6 Although the natural history of the disease tends often to remission with preservation of renal function, there is considerable morbidity and significant mortality,5,6 The risks of developing chronic renal failure, protracted disease course, and significant morbidity have pushed investigators to search for specific and effective therapy for this disease.
Various therapeutic approaches have been used for HBV-GN including corticosteroids, thymic extracts, acyclovir, interferon (IFN), and lamivudine. It has been argued that corticosteroid and immuno-suppressive agents are unfavorable for HBV-GN since they inhibit the immune system and activate latent HBV, finally lead to active replication of HBV and deterioration of renal lesions.7,8 Anti-viral drug has been recommended as the most important therapy of HBV-GN. However, the literature about antiviral therapy of HBV-GN was predominantly comprised by small clinical trials and case reports, and thus its efficacy remains poorly established.
The aim of our study is to evaluate the available evidences on tolerability and efficacy of anti-viral therapy in both of adult and pediatric patients by performing a systematic review of the literature with a meta-analysis of clinical trials.
Subjects and MethodsData Sources and SearchPubMed, Embase and Cochrane Library were searched using the crossed MESH terms: ‘hepatitis B virus’ or ‘hepatitis B’, ‘glomerulonephritis’ or ‘nephropathy’ or ‘membranous nephropathy’, ‘IFN’, ‘lamivudine’. All articles were identified by a search from June 1966 to October 2010. Bibliographies of relevant studies, reference list in ISI Web of Knowledge, proceedings of major recent meetings on nephrology and hepatology and “Related Articles” in PubMed were used to identify additional studies.
Identified abstracts and titles were screened independently by 2 investigators (Z Y., J.Y.W.) for inclusion in further analysis. All articles identified by the reviewers were retained. In the second round of screening, the full text for the articles was examined. The agreement between reviewers for article eligibility was 100% in second screening.
Criteria for inclusionControlled clinical trials, cohort studies, and case-control studies were enrolled in this systematic review. Inclusion criteria were:
- •
The diagnosis of HBV-GN was established based on renal biopsy.
- •
Studies reported the results of anti-viral (IFN or lamivudine) therapy of HBV-GN.
- •
The primary and secondary outcomes were remission of proteinuria (or clinical response) and clearance of hepatitis B e-antigen (orVR), respectively.
Exclusion criteria were:
- •
Patients in the studies had evidence of other secondary glomerulonephritis or other viral infections such as hepatitis C virus.
- •
Studies were on therapy of HBV-GN with Chinese herbal drugs.
- •
Studies reported inadequate data on measures of response.
The primary outcome in this systematic review was remission of proteinuria as a measure of efficacy. Clinical responses were divided into CR and PR, which were respectively defined as disappearance of proteinuria (< 0.3 g/d) and reduction in urine protein excretion. Secondary outcome was the clearance of hepatitis B e-antigen (HBeAg). Sustained clinical responses and VR were defined as remission of proteinuria and HBeAg clearance occurring over the follow-up period (at least 6 months after completion of therapy), respectively. These definitions are standards currently used in the scientific literature.
Data collection and quality assessmentTwo reviewers selected the studies independently, and extracted data and outcomes according to the inclusion criteria. We extracted data elements from each trial, including: type of study, sample size, mean age, dosage and duration of anti-viral (IFN or lamivudine) therapy and the duration of follow-up. For trials that did not provide complete data, we contacted the corresponding author for it.
The quality of the trials was evaluated using the Jadad score,9 including randomization, description of withdrawals and dropouts, and whether the trial is double blinded. The maximum score is 5. The quality of allocation concealment was graded as adequate (A), unclear (B), inadequate (C), or not used (D) according to the application of randomization.
Data analysis and synthesisThe primary outcome measurement was the pooled estimate of the risk ratio (RR) for HBV-GN who received anti-viral therapy compared to those who did not. We calculated the Mantel-Hansel risk ratio for assessing efficacy of anti-viral therapy on HBV-GN. The average effects and 95% confidence intervals (CIs) were obtained using a fixed-effects model. To assess heterogeneity across trials, we used the Cochrane statistic test, with a P value < 0.1 considered significant. Mild, moderate, and severe heterogeneity was defined by I2 values at > 25, 50, and 75%, respectively.
A funnel plot was used to assess the presence of publication and other reporting biases by plotting the standard error against the log risk ratio. Using Egger’s linear regression method, we examined the association between the study size and estimated therapy effects. P ≤ 0.05 was considered significant.
The P-value threshold for statistical significance was set at 0.05 for effect size. Egger’s linear regression was undertaken with Stata 11.0 (Stata Corp., College Station, TX). All other analyses were carried out with RevMan 5.0.25 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2008). The study was performed in accordance to the recommendations by Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) work-group.
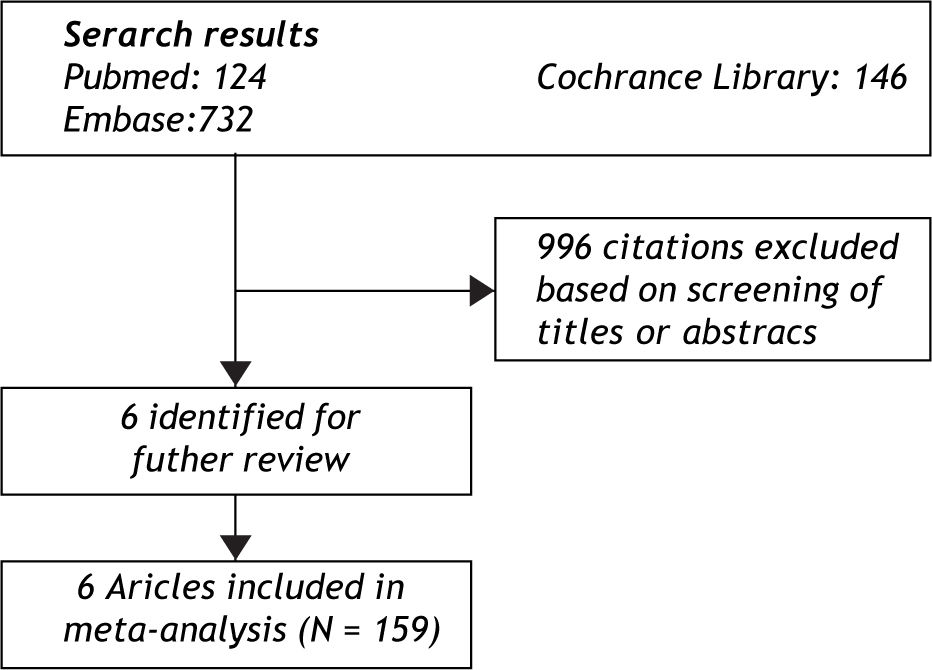
ResultsEligible studiesThe electronic search yielded 1002 citations (Figure 1). Of these, 6 clinical trials,10-1112131415 with a total of 159 patients, satisfied the inclusion criteria. Study characteristics were shown in Table 1. One of them was randomized controlled trial,10 others were cohort studies. Among the 6 articles, 4 (66.7%) were from China, corresponding to the high incidence of HBV-GN in China and the low incidence in Europe and North American. Three studies, with 82 patients, aimed at children (< 18 years old) while the other three aimed at adult patients. From a total of 159 patients, males were slightly predominant (2.25:1).
Baseline characteristic of clinical studies included in the analysis.
| Study | Case | Area | Age rang (Mean) | M:FF | Type of study | Jadad |
|---|---|---|---|---|---|---|
| Lin, et al. 1995 | 40 | Taiwan, China | <14ys(6) | 29M: 11F | Open-randomized trail | 3 |
| Rhimmal, et al.2002 | 39 | Africa | 5-14 ys(8.7) | 22M: 17F | Prospective, open-label observational study | 1 |
| Yang, et al.2003 | 33 | Wenzhou, China | 3-16ys(8) | 28M:5F | Corhort study | 1 |
| Tang, et al.2005 | 22 | Hong Kong, China | Adults(48) | 14M:8F | Corhort study | 1 |
| Panomsak, et al.2006 | 24 | Thailand | 23-74ys(43) | 14M:F10F | Corhort study | 1 |
| Lai, et al. 1991 | 21 | Hong Kong, China | 15-53ys(30) | 17M:4F | Corhort study | 2 |
M: Male. F: Female.
Study designs were shown in Table 2, which included histological type, the details of invention methods like dose and duration of anti-viral drugs, incidence of CR and PR, and duration of follow-up. The major histological type of 5 articles is MN while others included IgA nephropathy (IgAN), membranous nephropathy (MN), minimal change disease (MCD), (focal segmental glomerulosclerosis (FSGS) and mesangiocapilary proliferative glomerulonephritis (MPGN). Among the 159 patients (72 in treatment group with 5 dropped out, 87 in control group with 3 dropped out), 67 patients were on the therapy of anti-viral drugs and 84 patients were in the control group. IFN (1-10Mu tiw) by subcutaneous route was given in most of (2/3, 66.7%) clinical trials, and lamivudine therapy (100 mg qd) in two studies. The mean duration of follow-up was 3-108 months, which is variant between trials.
Design of 6 included clinical studies in the analysis.
| Study | N | Renal biopay | Antiviral therapy | CR | PR | VR | Follow up | Dropped-out |
|---|---|---|---|---|---|---|---|---|
| Lin, et al.1995 | 40 | MN | IFN-α 5-8Mu tiw *12w | 20/20 | 0 | 16/20 | 3 months | None |
| Rhimmal, et al.2002 | 39 | 36MN,2MP GS,1 NA | IFN-α 10Mu tiw*16w | 10/19 | 4/19 | 10/19 | 40 weeks | None |
| Yang, et al.2003 | 20 | MN | IFN 1 -3Mu tiw* (12w-24w) | 3/6 | 2/6 | 5/6 | 1 -6 years | 5 |
| Tang, et al.2005 | 22 | MN | Lamivudine 100 mgqd | 7/10 | 2/10 | 5/7 | 49.2±16.5 months | None |
| Panomsak, et al.2006 | 14 | IgA/MN/ FSGS/MPGS | 1 month of prednisone followed by IFN in 1 case or 6 lamivudine in 6case | 2/7 | 5/7 | 1/7 | 5-10 months | 3 |
| Lai, et al. 1991 | 16 | MN | 2 weeks of prednisone followed by rIFNα-2b 3Mu tiw | 1/5 | 4/5 | 1/5 | 12-108 months | None |
n: Numbers of the anti-viral therapy and the placebo. MN: Membranous glomerulonephriti. MPGN: Membranoproliferative glomerulonephritis. NA: Information needed not available. IFN: Interferon. CR: Complete remission. PR: Partial remission. VR: Virologic response.
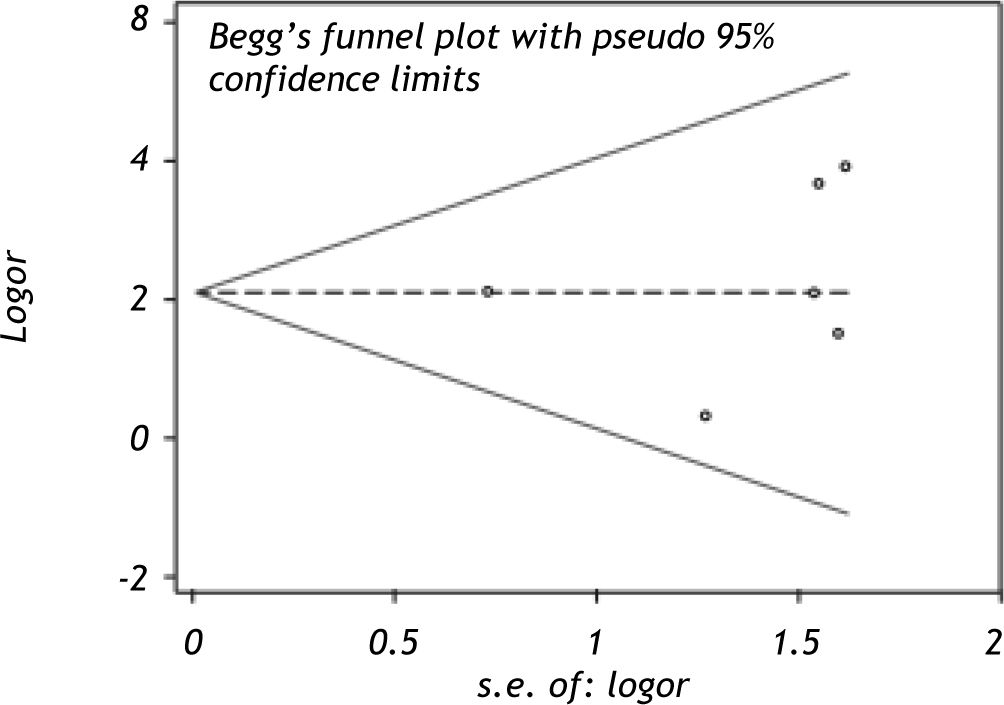
Visual inspection of the funnel plot (Figure 2) revealed symmetry, suggesting the absence of publication bias. Meanwhile, Egger’s test and begg’s test also support this observation (P = 0.704). It is concluded that publication bias may be acceptably low.
Efficacy of anti-viral therapy of HBV-GN in adult patientsSix studies were conducted in evaluation efficacy of anti-viral therapy on HBV-GN. The clinical manifestations and development of HBV-GN tend to be different in pediatric and adult patients,16 so we performed a sub-analysis between these two groups. The pediatric patient is defined as the patients whose age is 18 years old or younger, which was used in most trials.
Three studies121415 were conducted in adult patients. All trials were cohort studies. In Tang’s trial,12 10 HBV-MN patients had been treated with lamivudine (100 mg p.o., Q.D.). In Panomask’s study,14 patients with IgAN were treated with interferon-α2a and lamivudine in addition to an initial month of prednisolone therapy. Five patients in Lais’ study15 who had persisted proteinuria in the nephrotic range (> 3.0 g of urinary protein per day) for at least 12 months after diagnosis were randomly selected for the group of recombinant human interferon alfa-2b. These five patients first received a 2-week course of prednisolone (40 mg per day orally), followed by interferon therapy in a dose of 3 million units given subcutaneously three times a week for 12 weeks.
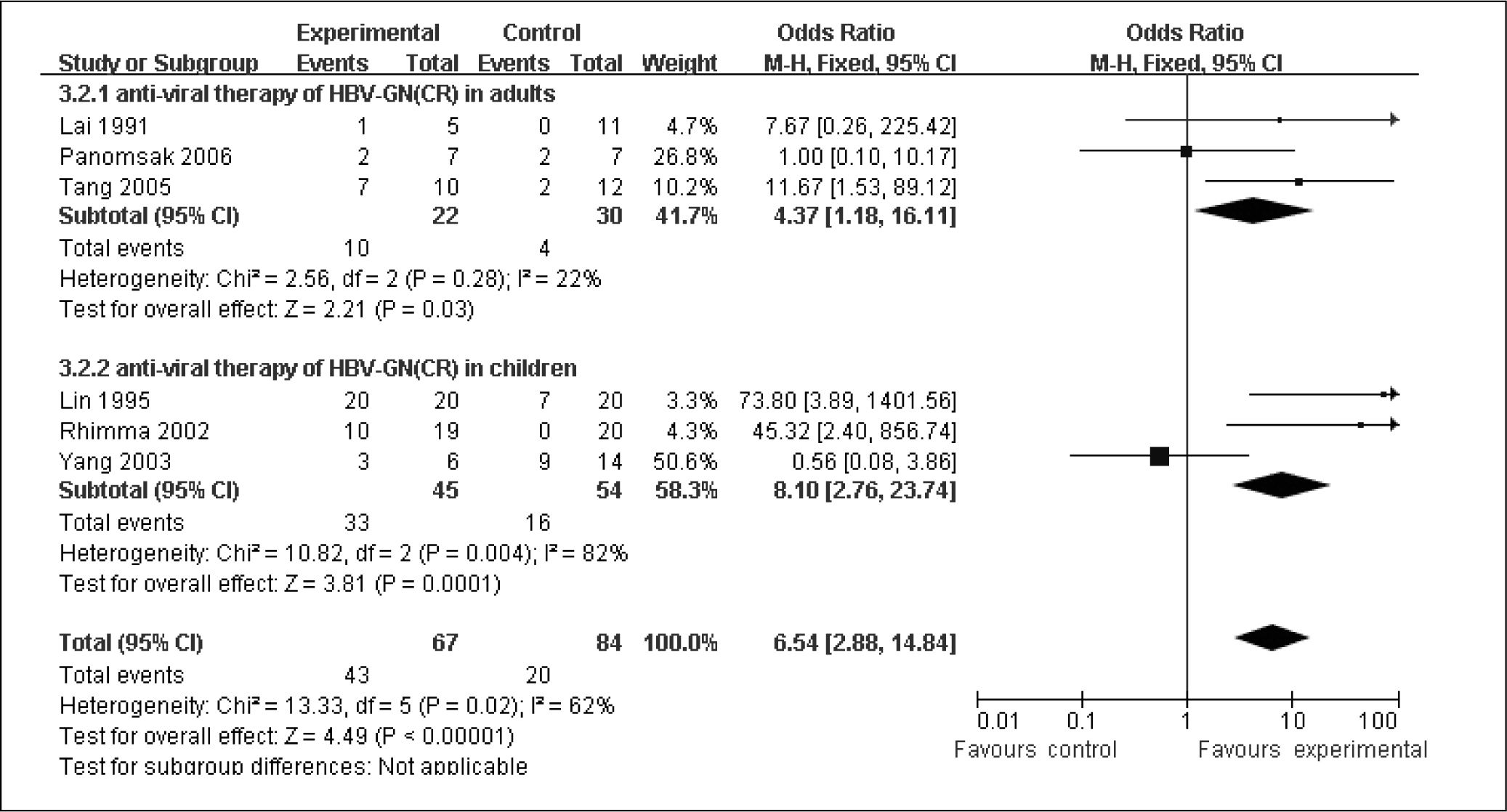
These three trails comprised 34.3% of the total 151 patients. The total number of CR and PR were 14 and 22, respectively. First, we evaluated the efficacy of anti-viral on total remission, which included CR and PR (Figure 3). In the absence of clinical and statistical heterogeneity (I2 = 0%, P = 0.57), the three trials were pooled. The pooled RR was 18.06 (2.97 to 109.93, P = 0.002), confirming the efficacy on clinical remission by anti-viral therapy on HBV-GN in adult patients. Secondly, we tended to know the anti-viral therapy on CR (Figure 4). The pooled RR was 4.37(1.18 to 16.11, P = 0.03) with no clinical and statistical heterogeneity (I2 = 21.7%, P = 0.28), emphasizing the good effect of anti-viral on CR in adult patients.
The other three studies101113 were conducted in pediatric patients, which comprised 65.7% of the total patients. The histological types in three studies were all HBV-MN. In Lin’s open, randomized trial study,10 all were pathologically proven HBV-MN and showed no response to corticosteroid therapy as they represented by a persistent heavy proteinuria. 20 HBV-MN patients were treated with recombinant IFNa (5 subjects, body weight < 20 kg; 8 subjects, body weight ≥ 20 kg) by subcutaneous (s.c.)injection three times a week for 12 months. In Rhimma’s prospective, open-labeled, observational study,11 IFNα 2b in a dose of 10 million units/m2 (maximum 10 million units/dose) was administered subcutaneously 3 times/week for 16 weeks. In Yang’s study,13 interferon was used to treat HBV-MN, compared with placebo group and prednisone group.
The pooled RR of the incidence of total remission in HBV-MN was 5.6 (1.77 to 17.75, P = 0.003) with no clinical and statistical heterogeneity (I2 = 0%, P = 0.45), which meant the good efficacy of anti-viral therapy in increasing the incidence of total remission in pediatric patients (Figure 3). However, in the presence of clinical and statistic heterogeneity (I2 = 81.5%, P = 0.004) in the analysis of CR, we could not pooled the efficacy of anti-viral therapy on CR (Figure 4).
Efficacy of anti-viral therapy of HBV-MN in adult patientsHBV-MN is the most common histological type in HBV-GN. In our study, we also performed a systematic review on efficacy of anti-viral therapy in HBV-MN patients. A sub-group analysis for pediatric patients was mentioned as above. We had been found that anti-viral therapy was benefit for increasing the incidence of total remission in pediatric patients. Then we made the same analysis in adult patients.
Five studies, included 137 patients, studied the efficacy of anti-viral therapy on HBV-MN. Two studies1215 were adult patients and the other three101113 were pediatric patients. Adult group included 38 patients, comprised 27.7% of the total 137 patients. In the absence of clinical and statistical heterogeneity in total remission evaluation (I2 = 0%, P = 0.33)(Figure 5) and CR evaluation (I2 = 0%, P = 0.83) (Figure 6), we pooled the efficacy that anti-viral is benefit for both total remission (RR = 14.52, 1.72 to 122.42) and CR of proteinuria (RR = 10.41, .82 to 59.39).
In order to evaluate the effect of anti-viral therapy on VR, we extracted the proportion of HBeAg seroconversion and the decline level of HBV-DNA, and made a systematic analysis (Figure 7). All the six studies had been included in the analysis. In the absence of clinical and statistical heterogeneity (I2 = 0%, P = 0.48), the six trials were pooled. The pooled RR was 15.14 (5.68 to 40.04, P < 0.00001), confirming the efficacy on HBeAg seroconversion by anti-viral therapy on HBV-GN. Two studies had showed the declined level of HBV-DNA. The pooled RR of the decline of HBV-DNA was 50.9 (5.60 to 463.16) with no clinical and statistical heterogeneity (I2 = 0%, P = 0.70), which meant the decline rate of HBV DNA in anti-viral therapy group was significant higher than control group (P = 0.0005). The results showed that VR kept with the remission of proteinuria.
DiscussionOur meta-analysis showed that most patients with HBV-GN were successfully treated by anti-viral therapy. The clinical remissions of pediatric and adult patients in therapy groups are significantly higher than control groups. CR is a more meaningful index in evaluating efficacy of anti-viral therapy. Then we divided the clinical remission into total remission and CR. We found that all of the adult patients who received anti-viral therapies were helpful for total remission and CR. In pediatric patients, anti-viral therapy was only benefit for total remission. Because of the existence of clinical and statistical heterogeneity in the analysis of CR in pediatric patients, we could not pool the results, which meant more trials should be carried out. Meanwhile, we also had found that the incidence of HBeAg seroconversion and the decline level of HBV-DNA were higher in therapy groups than in control groups, which kept with the remission of proteinuria. These results suggest that anti-viral therapy is of value in remission of proteinuria, and provides some benefit in seroconversion of HBeAg and clearance of HBV-DNA.
Some factors, such as the type of anti-viral drugs, may be related to the efficacy of anti-viral therapy. In our meta-analysis, 4 of 6 trials used IFN to treat HBV-GN; the other 2 trials used lamivudine. Among lamivudine-based trials, 2/3 had showed the benefit of lamivudine therapy. Among IFN-based trials, only 1/4 had showed the benefit. Hossein, et al.’s16 analysis of 119 patients from 10 studies also showed that lamivudine therapy was significantly associated with better outcome compared with IFN therapy; all patients receiving lamivudine had partial or complete remission, and none of them lost their kidneys. Moreover, when they repeated analysis with patients receiving only steroids and interferon, we did not find such a relationship.
Dosage and treatment duration may be another important factor impact the clinical outcome. Among lamivudine-based therapy, lamivudine (100 mg p.o., daily) for 12 months was commonly used. In Lai, et al.’s17 study, they suggested that long-term lamivudine therapy should be used only for patients who do not experience remission under supportive treatment, or for those with relapse of nephrosis after lamivudine withdrawal, which in turn, to prevent lamivudine-resistant mutations and hepatitis flare-ups. Moreover, cost related issues needs to be evaluated further. In IFN-based therapy, dosage and duration were not unified. Lin, et al. showed that IFNa therapy given by subcutaneous (s.c.) injection with less than 3 million units three times per week injection for six months was ineffective. In contrast, when the dose of IFNa was increased to 5 million units or given more than three times per week, the disappearance of proteinuria and HBeAg seroconversion was noted.18 Among the 4 INF-based trials included in our meta-analysis, only one trial, in which recombinant IFNa were increased to 5-8 million units, showed the significantly benefit of IFN therapy.
Like primate glomerulonephritis, different histological type had different efficacy to the therapy. In our meta-analysis, histological diagnosis in 5 trials was MN. Only pathologic types in Panomsak, et al.14 included IgA nephropathy (29%), MN (21%), FSGS (11%), membranoproliferative GN (11%), and postinfectious GN (11%). Remission rates both complete and partial in this trial were higher than in the literature with an average rate of 75% (30-60%), notwithstanding treatment. We thought that anti-viral therapy in IgA nephropathy had higher than MN; however, this point needs more trials to be verified.
Several questions merit investigation in this area. Most patients had different clinical characteristics such as magnitude of proteinuria, histological type, and age range; then could they be treated with the same therapy? We found trials in our analysis all included patients with proteinuria ranged from 3.0-10 g/day. Anti-viral therapy had played a major role in the remission of proteinuria and reduction of HBeAg and HBV-DNA. Still, more trials are needed to evaluate the efficacy of anti-viral therapy in HBV-GN patients with proteinuria < 3.0 and with different clinical presentation.
Lamivudine resistant-HBV mutation is a new problem, which could affect the efficacy of anti-viral therapy. Recently, the incidence of lamivudine resistant-HBV mutant strain is approximately 20% per year. In addition, recurrence of proteinuria after cessation of lamivudine is another problem.18 Entecavir is more preferable in long-term use because of lower incidence of drug-resistant mutation, 0.8% over 5 years.19 Takayasu, et al.’s20 case report was the first report on the clinical effects of entecavir in HBV-related MN showing HBeAg seroconversion and HBV clearance within 4 months, and subsequent complete remission of proteinuria. Entecavir may be replacement of lamivudine.
More attention should be given to the use of predinose. Among six studies, three studies had a drug history of short-term(2-4 weels) predinsolone, which may affect the relusts of anti-viral treatment. However, we thought short-term predinsolone had no response with the results, which is accord with Lin’s study.
Our meta-analysis has several limitations. First, the quality of a meta-analysis depends on the quality of the individual studies, which in this study was low. Second, the limited number of available studies made the conclusion with less force. Also, publication bias may pose a limitation. Third, Usually, it may not be easy to have neutral or negative studies published while positive studies do. Moreover, the time of treatment was not long enough to evaluate its effects on chronic HBV-GN.
ConclusionThe efficacy of antiviral therapy (including IFN and lamivudine) on HBV-GN is good. Antiviral therapy is effective on remission of proteinuria, HBeAg clearance, and HBV-DNA reduction. More randomized controlled trials should be taken to stratified the factors which be related to efficacy of anti-viral therapy.
DisclosuresNone.
Conflict of Interest StatementNone declared.