Previous studies found famine exposure was associated with a higher risk of metabolic syndrome (MetS). In the study, we investigated the relationship between Chinese famine exposure and the risk of nonalcoholic fatty liver disease (NAFLD) in adult women.
Materials and MethodsData were obtained from subjects via routine physical examinations in the Public Health Center of our hospital between 2011 and 2014. Women were categorized into the following three groups: control, pre-natally exposed, and postnatally exposed. Hepatic steatosis was diagnosed according to the guidelines established for the diagnosis and treatment of NAFLD.
ResultsThe prevalence rates of NAFLD among non-exposed, prenatally, and postnatally exposed women were 17.3, 23.0, and 22.9%, respectively. Pre-exposed and postnatally exposed women had higher risks of NAFLD, exhibiting ORs (95% CI) of 1.33 (1.04-1.70) and 1.26 (1.03-1.55), respectively. Prenatally, but not postnatally, exposed women had significantly higher risks of having abnormal alanine aminotransferase (ALT), with ORs of 1.30 (1.05-1.61).
ConclusionsThe results indicate a significant association between famine exposure in early life and the risk of NAFLD in adult women. Prenatally exposed women displayed higher risks of NAFLD and mild, moderate and severe steatosis.
Famine studies have supported the hypothesis that early malnutrition plays a role in the origins of metabolic syndrome (MetS).1-3 Li, et al.4 found positive associations between exposure to the Chinese famine during fetal life or infancy and increased risk of MetS in adulthood. These associations were stronger among subjects with Western dietary patterns and subjects who were overweight in adulthood. Our previous study5 found that exposure to the Chinese famine in early life was associated with increased risk of MetS in adult women, and famine exposure had stronger association with MetS when it was defined according to the ATPIII and IDF criteria which gave a pivotal role to central obesity.
Nonalcoholic fatty liver disease (NAFLD), the pathological accumulation of fat in the liver in the absence of alcohol intake, has become a most common chronic liver disease in China. The prevalence of NAFLD among adults in the general population in China is approximately 15% (6.3-27.0%), depending on the population studied in different geographical regions.6 If analyzed population resides in rural areas where they still present traditional diets and lifestyles, the reported NAFLD prevalence is about 10%. Nevertheless, if the analyzed people reside in urban areas where they have western lifestyles, the prevalence NAFLD is higher.7 Li, et al. found the prevalence of NAFLD is 6.3% among the 9,094 surveyed subjects in Chendu area in 2009.8 There is no exact data on the prevalence of NAFLD in Chongqing area; nevertheless, both Chongqing and Chengdu are in the Southwest China, people's living habits are basically the same. So, it can be concluded that the prevalence of NAFLD in Chongqing is similar to that reported by Li, et al.8
The etiology of NAFLD is multi-factorial. In the recent years it has been reported that NAFLD pathogenesis is related with a multi hits theory where insulin resistance (IR) has a central role and renders hepatocytes susceptible to other pathogenetic factors.9 NAFLD is the liver border of metabolic syndrome, can be associated to many metabolic changes.10 A combination of environmental and genetic factors determines an individual's risk of NAFLD development and progression, with nutrition clearly constituting a modifiable environmental risk factor. Recently, a wealth of evidence3-5,11-12 has accumulated suggesting that maternal nutrition can influence the susceptibility of offspring to developing components of MetS in adult life. Barker, et al.13,14 hypothesized that adverse environmental factors in early life may disrupt normal growth and development, a phenomenon that became known as the “developmental origins of health and disease” (DOHaD) hypothesis. Specifically, maternal malnutrition during the critical periods of development has been shown to prime adipose tissue deposition, leading to obesity later in life, particularly when the offspring is challenged postnatally with a hyper-nutritional diet.15 Taken together, these observations suggest that the maternal nutritional state and conditions during early development may lead to increased susceptibility to multifactorial disorders, such as MetS and NAFLD.
The 1959-1961 Chinese famine and associated births provide a natural experiment to evaluate the possible association between malnutrition in early life and the development of diseases in adult life,16,17 Previous studies have associated famine exposure in early life with the risk of MetS in adult life.4,5 To our knowledge, no study has examined the association between famine exposure and the risk of NAFLD in later life. Therefore, the purpose of the present study is to examine whether exposure to the Chinese famine during early life is associated with the risk of NAFLD in adult women.
Materials and MethodsWe used data from subjects who underwent routine physical examinations in the Public Health Center of the First Affiliated Hospital of Chongqing Medical University from 2011 through 2014. All women were Chongqing (Southwest China) urban residents who voluntarily participated in the routine physical examinations. Most of the women still present traditional diets of China (a high-carbohydrate and low-fat diet), and just a few people like western food. Most of them have good economic condition and medical insurance, and do not have health complaints before the medical checkup.
The grouping method used herein has been previously described in detail.5 In brief, female subjects born in 1963 or 1964 (no intrauterine or postnatal exposure) were classified as the control group; female subjects born in 1960 or 1961 (entirely intrauterine or prenatal exposure) were classified as the fetal exposure group; and female subjects born in 1957 or 1958 (entirely postnatal exposure) were classified as the postnatal exposure group. There is some uncertainty regarding the exact dates of the start and end of the Chinese famine. Thus, some women born in 1959 were likely exposed to the famine late in pregnancy, and some women born in 1962 were likely exposed to the famine in early pregnancy. Therefore, to minimize the mis-classification of the famine exposure periods, we excluded subjects born in 1959 and 1962. For convenience, we excluded subjects with missing data on any of the clinical laboratory parameters and anthropometric measurements including abdominal ultrasound examination results.
Anthropometric measurements included height, weight, waist circumference, and blood pressure. Height and weight were measured while the subjects were wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m). Waist circumference was measured at the midpoint between the bottom of the rib cage and the top of the iliac crest at the end of exhalation. Blood pressure, including systolic blood pressure (SBP) and diastolic blood pressure (DBP), was measured using each subject's right arm after a 5 min rest and from a sitting position. Blood biochemical analyses included cholesterol (TC), triglyc-erides (TG), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). All participants agreed to abdominal B-ultrasonog-raphy examinations. Additionally, lifestyle behaviors, including smoking, alcohol intake and physical activity, were surveyed using a questionnaire on health-related behaviors.
Abdominal ultrasonography was performed by experienced technicians who did not know the design of this study on HD7 ultrasound system (Philips, Shenyang, China). The diagnosis of NAFLD was according to the clinical diagnosis of the guidelines for the diagnosis and treatment of nonalcoholic fatty liver disease in 2008. In addition, the ultrasonographic results were differentiated into mild, moderate and severe cases of fatty liver according to the ultrasonic diagnosis of this guideline.18
The study was approved by the human research ethics committee of the First Affiliated Hospital of Chongqing Medical University.
Statistical analysesStatistical analyses were performed using SAS software (version 9.0; SAS Institute, Inc., Cary, North Carolina). Continuous variables with normal distributions are expressed as the mean ± standard deviation (X ± SD), and categorical variables are described as percentages (%); logarithmic transformations were applied to variables with skewed distributions. Multiple-group comparisons of means were performed using generalized linear models (GLMs). χ2 tests were used to compare percentages. Logistic regression was used to obtain odds ratios (ORs) of categorical variables (NAFLD, mild steatosis, moderate and severe steatosis, abnormal ALT, and abnormal AST). Multi-factor analysis of variance and survey logistic regression analysis were used to control for age, BMI, waist, and so on. All statistical analyses were two-sided, and p-values less than 0.05 were considered to be statistically significant.
ResultsThe final sample size for the analyses was 8,752. A total of 4,476 women (51.1%) were not exposed to famine during gestation or early childhood, 1,873 women (21.4%) were exposed to famine prenatally, and 2403 women (27.5%) were exposed to famine postnatally.
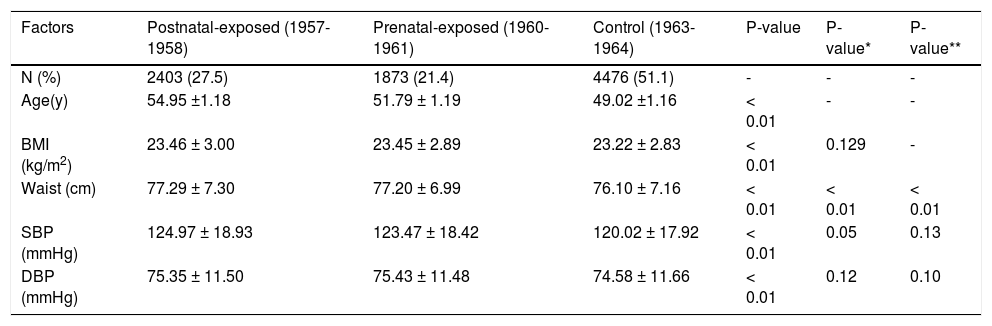
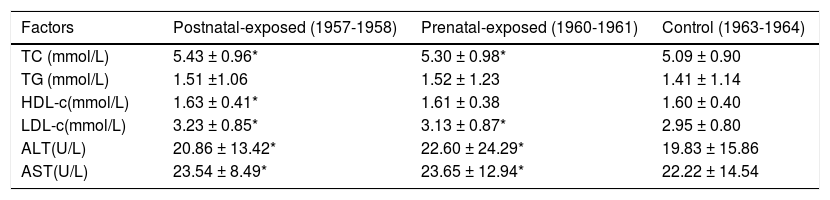
Compared to non-exposed women, prenatally and postnatally exposed women had significantly higher BMI, waist, SBP and DBP measurements (p < 0.01). After adjusted for age and BMI, only waist of prenatally and postnatlly women was significantly higher than those of non-exposed women (Table 1). Famine-exposed women exhibited significantly higher levels in their blood TC and LDL-c levels compared to non-exposed women, after adjusted for age, BMI and waist(p < 0.05). Famine-exposed women also had higher levels of ALT and AST compared to non-exposed women, after adjusted for age, BMI and waist (p < 0.01). Prena-tally exposed women were more likely to have higher levels of ALT and AST than postnatally exposed women; however, the differences were not significant (p > 0.05). Details are shown in table 2.
Demographic variable of the studied women.
| Factors | Postnatal-exposed (1957-1958) | Prenatal-exposed (1960-1961) | Control (1963-1964) | P-value | P-value* | P-value** |
|---|---|---|---|---|---|---|
| N (%) | 2403 (27.5) | 1873 (21.4) | 4476 (51.1) | - | - | - |
| Age(y) | 54.95 ±1.18 | 51.79 ± 1.19 | 49.02 ±1.16 | < 0.01 | - | - |
| BMI (kg/m2) | 23.46 ± 3.00 | 23.45 ± 2.89 | 23.22 ± 2.83 | < 0.01 | 0.129 | - |
| Waist (cm) | 77.29 ± 7.30 | 77.20 ± 6.99 | 76.10 ± 7.16 | < 0.01 | < 0.01 | < 0.01 |
| SBP (mmHg) | 124.97 ± 18.93 | 123.47 ± 18.42 | 120.02 ± 17.92 | < 0.01 | 0.05 | 0.13 |
| DBP (mmHg) | 75.35 ± 11.50 | 75.43 ± 11.48 | 74.58 ± 11.66 | < 0.01 | 0.12 | 0.10 |
BMI: Body mass index. DBP: Diastolic blood pressure: SBP: Systolic blood pressure. P value for differences between the groups exposed and unexposed to famine. * P-value based on logistical regression analysis after adjustment for age. ** P-value after adjustment for age and BMI.
Laboratory variables of the studied women.
| Factors | Postnatal-exposed (1957-1958) | Prenatal-exposed (1960-1961) | Control (1963-1964) |
|---|---|---|---|
| TC (mmol/L) | 5.43 ± 0.96* | 5.30 ± 0.98* | 5.09 ± 0.90 |
| TG (mmol/L) | 1.51 ±1.06 | 1.52 ± 1.23 | 1.41 ± 1.14 |
| HDL-c(mmol/L) | 1.63 ± 0.41* | 1.61 ± 0.38 | 1.60 ± 0.40 |
| LDL-c(mmol/L) | 3.23 ± 0.85* | 3.13 ± 0.87* | 2.95 ± 0.80 |
| ALT(U/L) | 20.86 ± 13.42* | 22.60 ± 24.29* | 19.83 ± 15.86 |
| AST(U/L) | 23.54 ± 8.49* | 23.65 ± 12.94* | 22.22 ± 14.54 |
Adjusted factors include age, BMI, and waist. ALT: Alanine aminotransferase. AST: Aspartate aminotransferase. HDL-c: High density lipoprotein-cholesterol. LDL-c: low-density lipoprotein-cholesterol. TC: Total cholesterol. TG: triacylglycerol. * P < 0.05 compared with post-famine group born in 1963-1964.
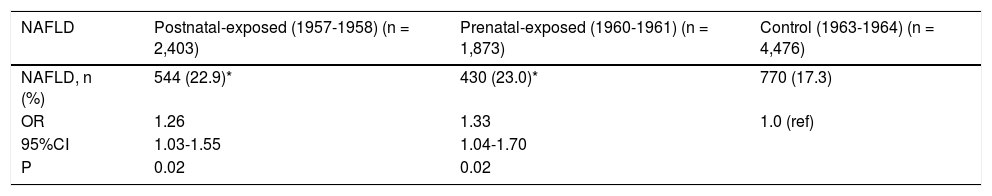
NAFLD prevalence rates among non-exposed, pre-natally exposed, and postnatally exposed women were 17.3, 23.0, and 22.9%, respectively. Women who were prenatally and postnatally exposed to the famine had higher risks of NAFLD compared to non-exposed women (odds ratio [OR] 1.33, 95% CI 1.04-1.70, p < 0.01; OR 1.26, 95% CI 1.03-1.55, p < 0.01, respectively), after adjusted for age, BMI, waist, blood pressure, blood lipids and enzyme levels. Prenatally exposed women were more likely than postnatally exposed women to have NAFLD. Details are shown in table 3.
The prevalence and ORs of NAFLD in 8,752 Chinese women born around the Great Chinese Famine in Chongqing.
| NAFLD | Postnatal-exposed (1957-1958) (n = 2,403) | Prenatal-exposed (1960-1961) (n = 1,873) | Control (1963-1964) (n = 4,476) |
|---|---|---|---|
| NAFLD, n (%) | 544 (22.9)* | 430 (23.0)* | 770 (17.3) |
| OR | 1.26 | 1.33 | 1.0 (ref) |
| 95%CI | 1.03-1.55 | 1.04-1.70 | |
| P | 0.02 | 0.02 |
CI: Confidence interval. NAFLD: Nonalcoholic fatty liver disease. ORs: Odds ratios. ORs based on logistical regression analysis after adjustment for age, BMI, waist, blood pressure, lipids and enzyme levels. * P < 0.05 Ccmpared with control cohort born in 1963-1964. All ORs use non-exposed cohort as reference cohort (ref).
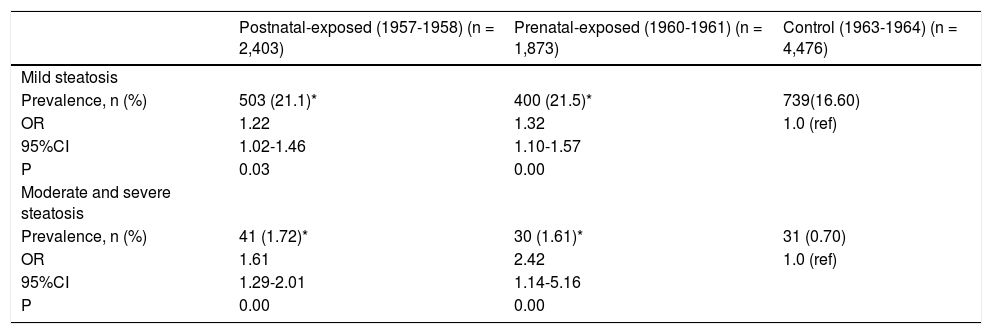
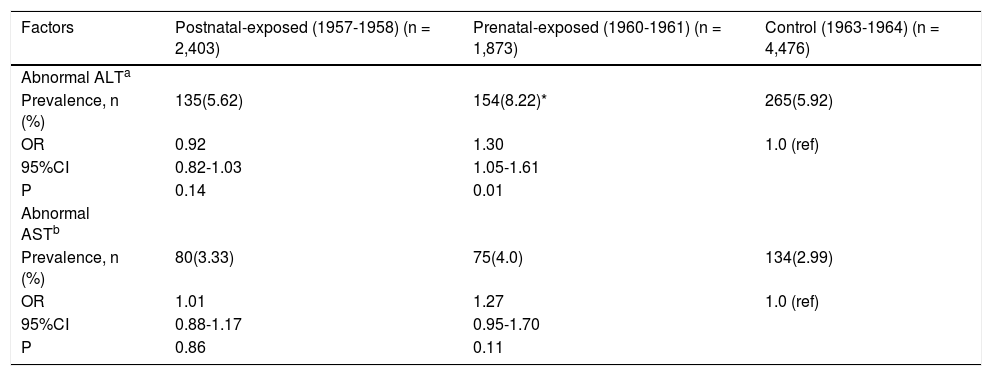
The severity of NAFLD can be categorized as mild, moderate, and severe steatosis according to ultrasono-graphical features. The prevalence rates of mild steato-sis among non-exposed, prenatally exposed, and postnatally exposed women were 16.6, 21.5, and 21.1%, respectively. Compared with non-exposed women, the risks of mild steatosis among women who were prena-tally and postnatally exposed to the famine were 1.32 (95% CI 1.10-1.57, p < 0.01) and 1.22 (95% CI 1.02-1.46, p < 0.01), respectively, after adjusted for age, BMI, waist, blood pressure, blood lipids and enzyme levels. Due to the relatively small number of severe grades of NAFLD, we combined the women with moderate and severe steatosis for further analyses. The prevalence rates of moderate and severe steatosis among non-exposed, prenatally exposed, and postnatally exposed women were 0.7, 1.6, and 1.7%, respectively. Compared with women who were not exposed to famine, the ORs were 2.42 (1.14-5.16) for prenatally exposed women and 1.61(1.29-2.01) for postnatally exposed women, after adjusted for age, BMI, waist, blood pressure, blood lipids and enzyme levels. As expected, women who were pre-natally exposed to the famine were more likely to have mild, moderate and severe steatosis. Details are shown in table 4.Comparing the prevalence rates and ORs among the experimental groups and defining abnormal ALT levels as values higher than 40 U/L, the prevalence of abnormal ALT levels in prenatally exposed women was significantly higher than that in non-exposed women (8.22% vs. 5.92%, OR 1.30, 95% CI 1.05-1.61, p = 0.01), after adjustment for age, BMI, waist, blood pressure, and blood lipids levels, while the prevalence of abnormal ALT levels was not significantly different between postnatally exposed and non-exposed women. When abnormal AST levels were defined as values higher than 40 U/L, the prevalence of abnormal AST levels in these groups was not significantly different. Details are shown in table 5.
The prevalence and ORs of mild and severe steatosis of the studied women.
| Postnatal-exposed (1957-1958) (n = 2,403) | Prenatal-exposed (1960-1961) (n = 1,873) | Control (1963-1964) (n = 4,476) | |
|---|---|---|---|
| Mild steatosis | |||
| Prevalence, n (%) | 503 (21.1)* | 400 (21.5)* | 739(16.60) |
| OR | 1.22 | 1.32 | 1.0 (ref) |
| 95%CI | 1.02-1.46 | 1.10-1.57 | |
| P | 0.03 | 0.00 | |
| Moderate and severe steatosis | |||
| Prevalence, n (%) | 41 (1.72)* | 30 (1.61)* | 31 (0.70) |
| OR | 1.61 | 2.42 | 1.0 (ref) |
| 95%CI | 1.29-2.01 | 1.14-5.16 | |
| P | 0.00 | 0.00 |
CI: Confidence interval. ORs: Odds ratios. * P < 0.05 compared with control cohort born in 1963-1964. All ORs use non-exposed cohort as reference cohort (ref). ORs based on logistical regression analysis after adjustment for age, BMI, waist, blood pressure, lipids and enzyme levels.
The prevalence and ORs of abnormal ALT and AST of the studied women.
| Factors | Postnatal-exposed (1957-1958) (n = 2,403) | Prenatal-exposed (1960-1961) (n = 1,873) | Control (1963-1964) (n = 4,476) |
|---|---|---|---|
| Abnormal ALTa | |||
| Prevalence, n (%) | 135(5.62) | 154(8.22)* | 265(5.92) |
| OR | 0.92 | 1.30 | 1.0 (ref) |
| 95%CI | 0.82-1.03 | 1.05-1.61 | |
| P | 0.14 | 0.01 | |
| Abnormal ASTb | |||
| Prevalence, n (%) | 80(3.33) | 75(4.0) | 134(2.99) |
| OR | 1.01 | 1.27 | 1.0 (ref) |
| 95%CI | 0.88-1.17 | 0.95-1.70 | |
| P | 0.86 | 0.11 |
ALT: Alanine aminotransferase. AST: Aspartate aminotransferase. CI: Confidence interval. ORs: odds ratios. a: Abnormal ALT is defined as ALT > 40 U/L. b: Abnormal AST is defined as AST ≥ 40 U/L. * P < 0.05 compared with control cohort born in 1963-1964. All ORs use non-exposed cohort as reference cohort (ref). ORs based on logistical regression analysis after adjustment for age, BMI, waist, blood pressure, and blood lipids.
This retrospective study analyzed the relationship between famine exposure in early life and the risk of NAFLD in adult women. Several interesting findings are reported herein.
This study found that women who were prenatally and postnatally exposed to a famine had higher risks of NAFLD. Famine-exposed women also had higher risks of developing mild, moderate and severe steatosis. NAFLD is well known to be the liver manifestation of MetS, and the development of NAFLD is strongly linked to obesity and insulin resistance.6 The earliest origins of NAFLD and other metabolic diseases may lie in early life, and potentially in utero. The transmission of metabolic phenotypes from mother to offspring may lead to the generational transfer of risk for a multitude of adverse metabolic outcomes, including NAFLD.19
The predominant theory regarding the developmental origins of NAFLD is that fetal exposure to gesta-tional over-nutrition leads to increased NAFLD risk in offspring.20-25 The proposed pathways for the intergen-erational transmission of NAFLD have been surmised. The gestational environment drives increased metabolic fuel delivery to the fetus, leading to fetal hepatic fat accumulation. Through epigenetic changes acquired prenatally, the liver is then “primed” for postnatal fat storage and inflammation mediated by Kupffer cells. A high-fat childhood diet triggers further steatosis, hepa-tocyte injury, inflammation, and fibrosis by activating hepatic stellate cells.26
Contrary to the above theory, our data indicate that fetal exposure to gestational malnutrition may also lead to increased NAFLD risk. Maternal malnutrition may result in a shortage of several crucial proteins, minerals and vitamins, which may be important for the early development of the fetal liver. For example, some stud-ies27,28 have suggested a role of vitamin D deficiency in the pathogenesis of NAFLD. In addition, manipulation of fetal energy supply (low protein) has been shown to modify the process of islet cell expansion, leading to a small β-cell mass at birth, which becomes even more pronounced when a protein-restricted diet is maintained until weaning.29 Similarly, deficiencies in vital materials may result in intergenerational transmission by epigenetic changes. Alimujiang, et al.30 recently found women exposed to the Chinese famine had risks of breast cancer according to hormone (estrogen and progesterone) receptor status. The estrogen/estrogen receptor had reciprocal effects of NAFLD prevention. Based on available clinical information, chronic liver disease appears to progress more rapidly in men than in women, and cirrhosis is predominately a disease of men and postmenopausal women. Estradiol is a potent endogenous antioxidant. So we suggest that the famine, malnutrition, or the associated lack of fruit and vegetable consumption in early life time may be related to lower level of estrogen or functional defect of estrogen receptor, which may contribute to the pathogenesis of NAFLD in adulthood.
Collectively, these data suggest that maternal nutrition may constitute a common origin of NAFLD; furthermore, the data support the notion that early development may “prime” increased susceptibility to the multifactorial disorder that becomes MetS and NAFLD in later life.
Unexplained elevations in ALT concentrations have been strongly associated with adiposity and therefore may indicate NAFLD.31 NAFLD accounts for approximately 80% of individuals with elevated liver enzyme concentrations, and an elevated ALT concentration is significantly correlated with portal inflammation, portal fibrosis, and perisinusoidal fibrosis.32 In addition, ALT concentrations have been shown to be more sensitive than AST concentrations, and ALT has been shown to be useful as a screening test for fatty liver.33 Therefore, the present study also investigated the association of famine exposure and abnormal ALT concentrations. Our data showed that prenatally but not postnatally exposed women had significantly higher risks of having abnormal ALT concentrations. Our results also indicated that prenatally exposed women displayed the highest prevalence and risks of NAFLD and mild, moderate and severe steatosis.
Famine exposure in different stages of early life may lead to adverse health effects in adulthood. Huang, et al.34 found that postnatal exposure during the first 2-3 years of life reduced height and increased BMI and hypertension, whereas exposure during pregnancy and infancy reduced BMI. Wang, et al.35 found that exposure to the Great Chinese Famine only during the first trimester of pregnancy, only during infancy or during both fetal development and infancy increased the risk of hypertension in adulthood.
We speculate that exposure to famine during gestation may be a critical period in “conditioning” the fetus to the poor intrauterine environment and may “program” the risk of NAFLD in later life. Organs such as the liver and pancreas are more vulnerable during what are known as the “critical periods” of rapid growth and development. Thus, famine exposure during a specific period of gestation may lead to problems associated with organ development. A recent study36 found that prenatal exposure to malnutrition leads to the reprogramming of postnatal brain gene expression and that associated epigenetic modifications contribute to this reprogramming. In short, prenatal famine exposure makes the liver more vulnerable to nutrient deficiencies and leads to the restriction of liver development, resulting in NAFLD in later life.
Our findings should be interpreted in the context of their potential limitations. First, the presence of NAFLD was assessed by experienced radiologists using abdominal ultrasonography instead of pathologic findings, and we have no information on the intra- or inter-observer reliability of ultrasonographic examinations. Second, only women were used to investigate the relationship between famine exposure and NAFLD risk; unfortunately, we did not have detail information such as dietary, alcohol and somking consumption of men, so, there were only women included in this study. Further investigation is required to determine the risk of NAFLD in famine-exposed men. Third, compared to other famine studies, our sample size was relatively large, but our data only included information obtained from the Chongqing district rather than data from the entire country. Finally, most of the women selected in our study were urban residents. The effect of famine on rural residents requires further study.
ConclusionIn summary, we showed a significant association between famine exposure in early life and the risk of NAFLD in adult women. Prenatal famine exposure may condition the liver to be more vulnerable to nutrient deficiencies and lead to restricted liver development, resulting in NAFLD in later life.
Abbreviations- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
BMI: body mass index.
- •
CI: confidence interval.
- •
DBP: diastolic blood pressure.
- •
DOHaD: developmental origins of health and disease.
- •
GLMs: generalized linear models.
- •
HDL-c: high density lipoprotein-cholesterol.
- •
LDL-c: low-density lipoprotein-cholesterol.
- •
MetS: metabolic syndrome.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
NASH: nonalcoholic steatohepatitis.
- •
ORs: odds ratios.
- •
SAS: statistical analysis system.
- •
SBP: systolic blood pressure.
- •
SD: standard deviation.
- •
TC: total cholesterol.
- •
TG: triglyceride.
Xiaoya Zheng wrote manuscript, Lilin Gong and Jian Long researched data. Yonghong Wang, Wei Ren, and Rong Luo reviewed and edited manuscript.
AcknowledgmentsI hereby express gratitude to my dear Prof. Yong-hong Wang, without her effort, this thesis cannot be accomplished. Besides, she is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This thesis is rather a common achievement than a private possession.
SupportThis study was supported by the Chongqing municipal health and Family Planning Commission Fund (Number: 2016MSXM006), the Project of Chongqing science and Technology Committee (Number: cstc2016jcyj A0025), the Doctoral Fund of the Ministry of Education (Number: 20135503120001), the Beforehand Research Project of the National Natural Science Fund (Number: NSFYY201312), and the National Key Clinical Specialist Construction Project (2011). We thank all participants, particularly our hospital's data collection team.
Conflicts of InterestAll authors declare no conflicts of interest.