To evaluate the diagnostic value of dynamic contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) in the differential diagnosis of hepatic angiomyolipoma (HAML) and hepatocellular carcinoma (HCC) and to clarify the relationship between histopathological features and CT or MRI imaging performances in HAML.
Material and methodsSix HAML and 33 non-cirrhotic HCC patients confirmed by histopathology were retrospectively analyzed. The serum biomarkers, CT and MRI examinations were conventionally performed before the confirmatory histological diagnosis. The clinical data from their medical records was also analyzed.
ResultsSix HAML patients were annotated as two types according to CT and MRI imaging characteristics, including hypovascular type (n = 1) and hypervascular type (n = 5). The imaging performances of the 33 HCC patients were hypervas-cular type. Moreover, all the 5 hypervascular type HAML patients were misdiagnosed as HCC by CT or MRI. We also found that the hypervascular type of HAML patients contained more vessels and less fatty tissues in histopathology than hypovascular type of HAML patients. However, the clinical features included HCC high risk factors (hepatitis B or C), non-specific symptoms, male and increased serum alpha fetoprotein (AFP) were more common in HCC patients than HAML patients (P < 0.05, respectively).
ConclusionsThe CT or MRI imaging performances of HAML patients containing more vessels and less fatty tissues in histopathology resemble the imaging performance of HCC patients. These clinical features may be of great help in the differential diagnosis in the current clinical practices.
Hepatic angiomyolipoma (HAML) is one of the rare hepatic benign tumors and occurs in females more frequently, which is characterized by the composition of lipomatous, myomatous and angiomatous tissues.1 Most of HAML patients are asymptomatic when they are diagnosed. Clinically, HAML and primary hepatocellular carcinoma (HCC) are often indistinguishable. According to ACG clinical guideline,2 focal liver lesions in patients with liver cirrhosis should be considered a possibility of HCC. However, according to this criterion it was very difficult to distinguish HCC from liver benign tumors, particularly in those patients without liver cirrhosis.
The dynamic enhanced computed tomography (CT) and magnetic resonance imaging (MRI) are very useful imaging techniques in differentiating benign lesions from malignant tumors.2,3 However, the diagnostic value of CT and MRI in discriminating HAML and HCC is unde-fined.4 Hence, the aim of this study was to investigate the diagnostic value of CT and MRI in differential diagnosis of HAML and HCC in patients without cirrhosis, and to correlate the histopathological features to the CT or MRI imaging performances.
Material and MethodsPatientsThis was a retrospective study approved by Ethics Committee of Beijing Youan Hospital affiliated with Capital Medical University. A total of 40 nodule lesions in 6 HAML and 33 HCC patients were retrospectively analyzed. All enrolled HCC patients received surgery treatments from 2009 to 2014. The age of 6 HAML patients ranged from 30 to 66 years old. Four patients were females and the others were males. HCC candidates were enrolled according to the following criterion:
- •
HCC without cirrhosis, confirmed histologically.
- •
Available CT or MRI records obtained 1 month prior to the surgery.
- •
No history of interventional therapy such as radiotherapy, transcatheter arterial chemoembolization chemotherapy (TACE).
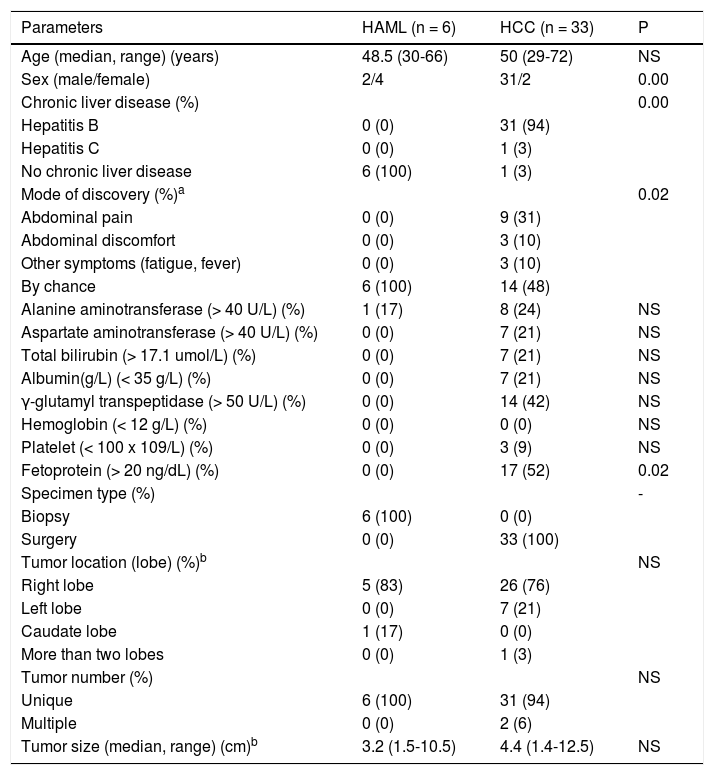
The ages of 33 HCC patients ranged from 29 to 72 years-old. Thirty-one patients were males and 2 patients were females. Of 33 HCC patients, 31 had chronic hepatitis B, 1 had chronic hepatitis C. The clinical data including demography, medical history, clinical manifestations, laboratory tests and tumor information were noted from their medical records (Table 1).
Clinical characteristics of all enrolled patients.
| Parameters | HAML (n = 6) | HCC (n = 33) | P |
|---|---|---|---|
| Age (median, range) (years) | 48.5 (30-66) | 50 (29-72) | NS |
| Sex (male/female) | 2/4 | 31/2 | 0.00 |
| Chronic liver disease (%) | 0.00 | ||
| Hepatitis B | 0 (0) | 31 (94) | |
| Hepatitis C | 0 (0) | 1 (3) | |
| No chronic liver disease | 6 (100) | 1 (3) | |
| Mode of discovery (%)a | 0.02 | ||
| Abdominal pain | 0 (0) | 9 (31) | |
| Abdominal discomfort | 0 (0) | 3 (10) | |
| Other symptoms (fatigue, fever) | 0 (0) | 3 (10) | |
| By chance | 6 (100) | 14 (48) | |
| Alanine aminotransferase (> 40 U/L) (%) | 1 (17) | 8 (24) | NS |
| Aspartate aminotransferase (> 40 U/L) (%) | 0 (0) | 7 (21) | NS |
| Total bilirubin (> 17.1 umol/L) (%) | 0 (0) | 7 (21) | NS |
| Albumin(g/L) (< 35 g/L) (%) | 0 (0) | 7 (21) | NS |
| γ-glutamyl transpeptidase (> 50 U/L) (%) | 0 (0) | 14 (42) | NS |
| Hemoglobin (< 12 g/L) (%) | 0 (0) | 0 (0) | NS |
| Platelet (< 100 x 109/L) (%) | 0 (0) | 3 (9) | NS |
| Fetoprotein (> 20 ng/dL) (%) | 0 (0) | 17 (52) | 0.02 |
| Specimen type (%) | - | ||
| Biopsy | 6 (100) | 0 (0) | |
| Surgery | 0 (0) | 33 (100) | |
| Tumor location (lobe) (%)b | NS | ||
| Right lobe | 5 (83) | 26 (76) | |
| Left lobe | 0 (0) | 7 (21) | |
| Caudate lobe | 1 (17) | 0 (0) | |
| More than two lobes | 0 (0) | 1 (3) | |
| Tumor number (%) | NS | ||
| Unique | 6 (100) | 31 (94) | |
| Multiple | 0 (0) | 2 (6) | |
| Tumor size (median, range) (cm)b | 3.2 (1.5-10.5) | 4.4 (1.4-12.5) | NS |
The enhanced CT imaging (Lightspeed 64 VCT; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) was conventionally performed through injecting Iopromide (Ultravist Injection) intravenously. The methods and scanning parameters were described by Li JJ, et al.5 The scanning parameters were as follows: 120 kV; 250-300 mA; 0.5 s/rotation; thickness, 5.0 mm; intersection gap, 5.0 mm; helical pitch (beam pitch), 0.984; and matrix: 512 x 512. Iopromide (370 mg/mL, Bayer Schering Pharma AG, Germany) was administered with dose of 1.5 mL/kg at an injection rate of 3 mL/s for a three-phase enhanced scan. Arterial phase imaging was started at 25~30 seconds after the injection of contrast medium, portal phase imaging and delayed phase imaging were started at 60~70 seconds, 180~300 seconds respectively.
MR imagingThe MRI scanning System (3.0 T, 32 Channel, SI-MENS MAGNETOM Trio A Tim System, German) was used. Dynamic scanning was performed as the patient held his breath by three dimensional volume interpolated body examination (VIBE) T1 weighted imaging (TR 3 .5 ms, TE 1. 25 ms, flip angle 9°, section thickness 3-3.5 mm, intersection gap 0.6-0.7 mm, rectangular field of view 380 mm x 285 mm, matrix 320 mm x 256 mm). The scanning time was from 18 to 25 s (median 22.5 s). Contrast agents (at a dosage of 0.1 mmol/kg) were injected by bolus tracking method at a rate of 2 mL/s, immediately followed by a 15-20 mL saline flush using an injector (Sonic shot GX, Nemoto Kyorindo Co., Ltd. Tokyo, Japan). The scanning was conventionally conducted before and after administration of Gadobenate dimeglumine (Gd-BOPTA, BRACCO PHARMA, Shanghai, China). The CT/MRI imaging was analyzed by two radiologists independently.
Histopathological analysis- •
HE staining. Liver tissue specimens from surgical excised and needle biopsies was fixed in 4% poly-oxymethylene and embedded in paraffin from 33 HCC and 6 HAML patients. The liver tissue samples for HE staining was cut at 4 μm sections on a rotary microtome and staining was performed using an automated HE stainer (Leica Autostainer XL, UK).
- •
Immunohistochemistry (IHC). Expressions of GPC3, CD34, hepatocytes, HMB-45 and SMA in liver tissue samples cut at 3 μm sections was detected with IHC staining. IHC staining was performed using an automated immunostainer (Leica Bond-Max, Milton Key-nes, UK). All sections were visualized with diaminobenzidine, counterstained with hematoxylin, and mounted in DPX. The CD34, hepatocytes, HMB-45, SMA (Monoclonal No. M716529, IR624, IR052, M0851, Dako, Denmark) and GPC3 (sc-17613, Santa Cruz, USA) primary monoclonal antibodies were diluted at 1:50 for IHC. The secondary antibody was horse-radish peroxidase (HRP)-labeled antibody (Zhongshan Golden Bridge, Beijing, China). The result was evaluated by the pathologists from the pathology department of Beijing Youan Hospital. Five high visual fields were observed with no less than 1,000 cells in each visual field. Cells in which the cytoplasm contained yellow or brown particles were considered positive.
All the analyses were performed using the SPSS for Windows (version16.0, IBM Corporation, ARMONK, NY USA). The data were expressed as mean ± SD. Mann-Whitney U and Fisher exact test were used to compare the distribution of continuous and categorical variables, respectively, in the two groups. P < 0.05 was considered statistically significant.
ResultsClinical characteristics of HAML and HCCCompared to the HCC patients, asymptomatic single nodule and normal serum AFP were more common in the HAML patients. However, high risk factors of HCC, more non-specific symptomatic nodule and higher serum AFP were more frequently found in HCC patients (Table 1). Furthermore, women had high tendency to get HAML and men had high tendency to get HCC.
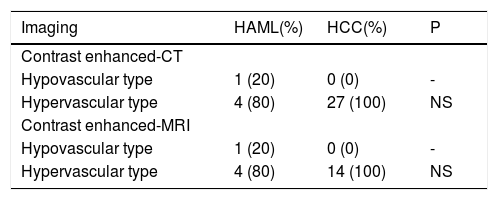
CT and MRI featuresOf the 39 patients, 5 HAML and 27 HCC patients had the records of contrast enhanced-CT. At the same time 5 HAML and 14 HCC patients had the records of contrast enhanced-MRI. In pre contrast enhanced-MRI, only one HAML patient showed high signal both on T1-weighted and T2-weighted imaging. However, the low signal on opposed phase T1-weighted imaging and mixed low and slightly high signal on fat-suppression T2-weighted imaging were observed in this patient. The other HAML patients and all HCC patients showed the same features, including low signal on T1-weighted imaging and high signal on T2-weighted imaging. Based on the dynamic CT and MRI performances, there were two mode types. First was the hypovascular type, in which tumor region was a weak and an unremarkable enhancement in all the periods. The second was the hypervascular type, which showed enhancement in the arterial phase and washout in the portal phase or delayed phase. Of the 5 HAML patients, 1 was hypovascular type and 4 were hypervascular type. All HCC nodule lesions showed hypervascular type. There were no significant differences between HAML and HCC groups in dynamic CT and MRI enhancement modes (P > 0.05) (Table 2).
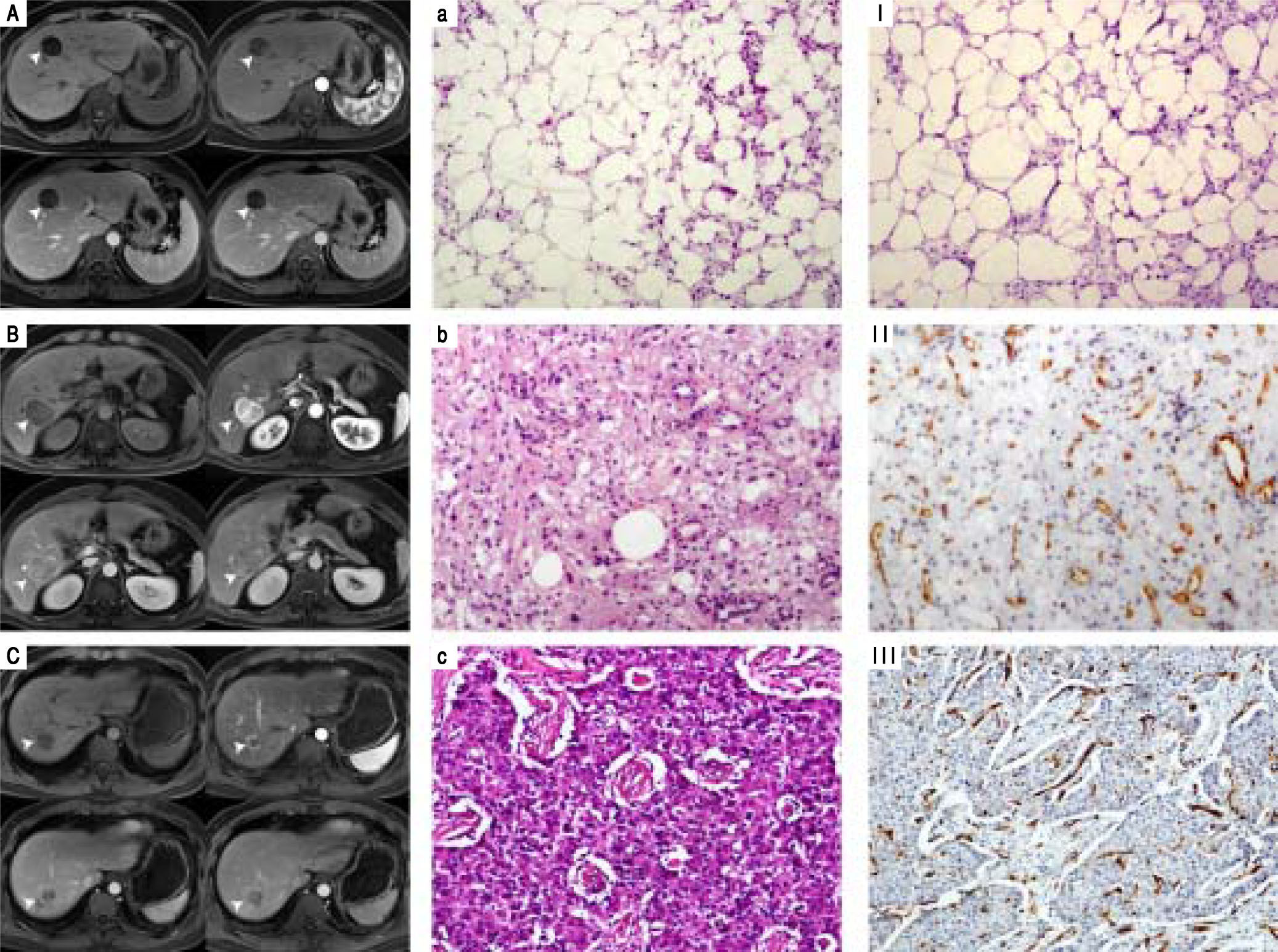
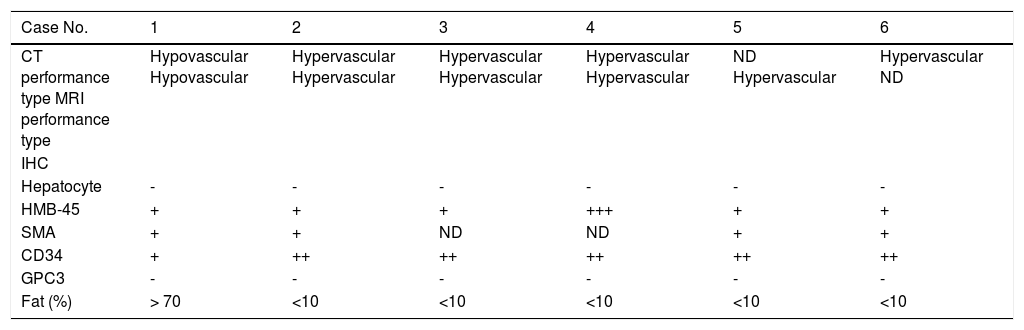
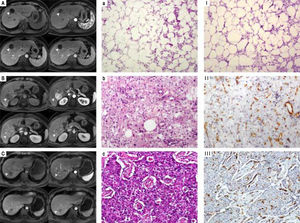
Histopathological features and CT/MRI imaging performancesThe CT/MRI imaging characteristics were related to the pathological features (Table 3). All the HAML patients were diagnosed by histopathology composited of blood vessels, smooth muscle cells and fat tissues. However, the proportion of these three components was not the same. All the 5 hypervascular HAML patients were misdiag-nosed as HCC by CT or MRI. But the other hypovascular patient was correctly diagnosed. We also found that the tumor of HAML misdiagnostic patients (hypervascular type) contained less fat tissues and more vessels than those of hypovascular type (Figure 1).
Relationship between CT/MRI imaging performances and histopathology in 6 HAML patients.
| Case No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| CT performance type MRI performance type | Hypovascular Hypovascular | Hypervascular Hypervascular | Hypervascular Hypervascular | Hypervascular Hypervascular | ND Hypervascular | Hypervascular ND |
| IHC | ||||||
| Hepatocyte | - | - | - | - | - | - |
| HMB-45 | + | + | + | +++ | + | + |
| SMA | + | + | ND | ND | + | + |
| CD34 | + | ++ | ++ | ++ | ++ | ++ |
| GPC3 | - | - | - | - | - | - |
| Fat (%) | > 70 | <10 | <10 | <10 | <10 | <10 |
NS: no significance. ND: not done. Cells in cytoplasm taking on yellow or brown particles were considered positive. ‘-’: no positive staining. ‘+’: positive staining was weak yellow. ‘++’: positive staining was yellow. ‘++’: strong positive staining was brown.
The relationship of histopathological features and CT/MRI imaging performances. A. Hypovascular type of HAML showed a weak and unremarkable enhancement (arrow) in all periods. B, C. Hypervascular type of HAML and HCC showed enhancement in the arterial phase (arrow) and washout in the portal phase or delayed phase. Accordingly, histopathological features of hypovascular type of HAML contained more fatty tissues and less vessels (a, I) than hypervas-cular type HAML (b, II) and HCC(c, III). a-c: HE staining, x100; I~III: CD34 IHC staining, x100.
As we known, CT and MRI examination are very useful in differentiating liver benign nodules from malignant tumors. However, a series of case reports indicated that the HAML, one of the rare hepatic benign tumors, was very difficult to differentiate from HCC. It is reported that the accurate preoperative diagnosis rate of HAML was only 25%-52%1 or even lower.6 Chang Z, et al.7 reported that the diagnosis of HAML by CT and MRI was not sensitive in a retrospective multicenter analysis of 178 patients. Kim R, et al.8 found similar enhancement patterns of HAML and HCC during the dynamic phases of MRI. Therefore, the diagnosis of HAML remains inconclusive by CT and/or MRI and mainly relies on pathological findings. Some studies have also summarized the imaging characteristics of HAML. Yang X, et al.9 had summarized the features of ultrasonic imaging in the non-cirrhotic focal liver lesions of HAML. They observed hyper-enhancement in the arterial phase which remains hyper-enhancement or iso-enhancement in the late phase. Cai PQ, et al.10 retrospectively analyzed the data of 18 cases of HAML and found the early draining vein, peripheral decreasing enhancement rim and the absence of tumor capsule in the hypervascular hepatic tumor of CT or MRI imaging performances.
In our data, one HAML patient showed hypovascular type by CT and MRI imaging performances. In that case, it was relatively easy to differentiate HAML from liver cancer. However, studies related to hypovascular HAML were seldom reported and hypervascular HAML were focused on by researchers.11 In this study, 5 of 6 (83.3%) HAML and all the 33 HCC (100%) patients showed hypervascular type by CT and/or MRI imaging performances. Further, all 5 hypervascular type HAML patients were misdiagnosed as HCC because of the similar CT and/or MRI imaging characteristics with HCC. HAML is composed of blood vessels, smooth muscle cells and a varying amount of fat histologically. Based on the line of differentiation and predominance of tissue components in his-topathology, these tumors have been classified into 4 subtypes: mixed, lipomatous (70% fat), myomatous (10% fat), and angiomatous. The most common subtype is the mixed subtype that comprises sheets of epithelioid muscle cells admixed with islands of adipocytes and abnormal vessels. The lipomatous and myomatous patterns are regarded as morphologic variations on a continuous spectrum depending on the degree of adipose and myoid differentiation. The myomatous subtype is more common in the liver than in the kidney. Angiomatous HAML contains many large thick-walled vessels and radiologically may be misinterpreted as an intrahepatic arterial aneu-rysm. Here we also confirmed that hypovascular type HAML was rich in fat tissue (> 70%) and contained less blood vessels. Moreover, hypervascular type HAML contained less fat tissue (< 10%) and was rich in blood vessels.
Notably, in the diagnosis of fat-containing lesions, MRI can provide more useful information than CT. However, it was reported that 19.6% of HCC patients were with fat-containing.12 Thus, it is important to use the enhanced MRI imaging or lesion biopsy to identify benign and malignant tumors.
To distinguish HAML and HCC in clinical practice, we must also pay more attention to the clinical information. In this study, we found that asymptomatic single nodule and normal serum AFP were more common in the HAML patients. On the other hand, HCC risk factors, such as hepatitis B or C virus infections, more non-specific symptomatic nodule and higher serum AFP were more frequently detected in HCC patients. Also, women had high tendency to develop HAML and men had high tendency to get HCC. These clinical features may be of great value in the differential diagnosis in the current clinical practices. To summarize for hypervascular type HAML, it is very difficult to differentiate HAML from HCC using current CT or MRI imaging techniques and we must pay attention to clinical information in clinical practices.
Abbreviations- •
AFP: alpha fetoprotein.
- •
AFU: α-L-fucosidase.
- •
ALT: alanine aminotransferase.
- •
CT: computed tomography.
- •
GPC3: glypican 3.
- •
HAML: hepatic angiomyolipoma.
- •
HCC: hepatocellular carcinoma.
- •
HMB-45: human melanoma black-45.
- •
MRI: magnetic resonance imaging.
- •
NS: not significant.
- •
SMA: smooth muscle actin.
The authors declared no conflict of interests.
AcknowledgementsThis study was supported by the Capital Science and Technology Development Fund (2014-1-2181), Beijing Municipal Administration of Hospitals’ Clinical medicine Development of special funding (ZYLX2016-10), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20151602) and National Natural Science Foundation (81672725). The authors also acknowledge Dr. Shi Qi and Hongjun Li, Department of Radiology in Beijing Youan Hospital, for their kindly help in data collection and imaging analysis.