Background. Though there is considerable evidence that sphingosine kinase 1(SPHK1) plays a key role in hepatocellular carcinoma(HCC) progression, the prognostic value of SPHK1 expression in HCC with portal vein tumor thrombus (PVTT) remains unclear.
Aims. The purpose of this study was to investigate the relationship of SPHK1 expression with PVTT and HCC recurrence after hepatectomy.
Methods. After screening of gene expression profiling of tumor cell lines, real-time PCR and immunohistochemistry were used to investigate the SPHK1 expression in PVTT and HCC samples. The clinical data of 199 HCC patients with nonmain PVTT who underwent liver resection with curative intention were studied.
Results. We identified SPHK1 as the most over-expressed gene in PVTT via gene expression profiling of one human PVTT cell line (CSQT-2). SPHK1 expression was an independent factor affecting survival (hazard ratio [HR] 1.799, 95% confidence interval [CI] 1.337-2.368, P < 0.001) and tumor recurrence (HR 1.451, 95% CI 1.087-1.935, P = 0.011). Patients with SPHK1 over-expression had a poorer prognosis than those with SPHK1 under-expression (P < 0.001 and P = 0.011 for survival and tumor recurrence).
Conclusions. SPHK1 might represent a novel and useful prognostic marker of HCC progression in patients with PVTT.
Hepatocellular carcinoma (HCC) tends to invade the intrahepatic vasculature, especially the portal vein. Portal vein tumor thrombus (PVTT) can be detected in 30.0% to 62.2% of patients with HCC.1 The presence of tumor thrombus is correlated with poor prognosis.2,3 The natural history of untreated HCC with PVTT is very poor. The median survival of patients with microscopic PVTT was reported to be 2.7 months, whereas the median survival of patients without PVTT was 24.4 months.4 Furthermore, portal vein invasion is correlated with intrahepatic metastasis and recurrence after treatment.3 PVTT grows very fast probably because it contains more microvessels in order to get more blood supply. The data indicated that the microvessel density of PVTT is more than that of the primary tumor.
The mechanism of angiogenesis of PVTT itself is rarely to be studied. Multiple lines of evidence indicate that SPHK1 is an oncogenic enzyme and that the activation of SPHK1 is closely associated with cell proliferation, migration, invasion, and angiogenesis.5–7 SPHK1 is responsible for the conversion of sphingosine to sphingosine 1-phosphate (S1P).8 Many growth and angiogenic factors involved in tumorigenesis may use S1P as their downstream signal transducer through SPHK1 activation.9 Indeed, high levels of SPHK1 expression and activity are associated with a poor prognosis in breast, glioma, and gastric cancers.10–12 The purpose of this prospective study is SPHK1 is an effective prognostic marker for HCC patients with PVTT.
Material and MethodsPVTT staging systemWe use the PVTT staging system which we established before.13,14 According to the extent, PVTT was divided into 4 types (I-segmental/sectoral branches of portal vein, II-left and/or right portal vein, III-main portal vein trunk, and IV-superior mesenteric vein).
Patients and tissue samplesOne hundred and ninety-nine patients HCC patients with different stages of macroscopic PVTT diagnosed by preoperative imaging were recruited between January 2000 and January 2004. All of the 199 patients were stratified according to stages I-II PVTT classification,13,14 and these patients met the following inclusion criteria and thus underwent the issue microarray (TMA) analysis:
- •
Distinctive pathological diagnosis of HCC.
- •
Surgical resection, defined as a complete resection with the cut surface being free of cancer by histologic examination.
- •
Complete clinicopathologic and follow-up data.
The exclusion criteria of the patients include:
- •
Having extrahepatic spread.
- •
Having prior anti-cancer treatment before liver resection.
- •
Having main-portal trunk PVTT.
An additional 27 HCC patients with macroscopic PVTT (81 samples) were recruited between January 2008 and January 2011, and their resected samples were subjected to RT-PCR verification and immunohistochemistry verification.
The curative resection of the HCC was performed as described.15 First, all detected lesions were resected, and an intraoperative ultrasound examination revealed no remnant tumor. Second, the negative surgical margins were confirmed by way of histological examination. Thrombectomy was performed according to the location and the extent of the PVTT. For patients with a PVTT located within the resected area, the PVTT was resected en bloc with the tumor. For patients with a PVTT beyond the resection line, the PVTT was extracted from the opened stump of the portal vein. After flushing with normal saline and confirming that no PVTT remained, the stump was closed with a continuous suture.
All patients received the same post-operative care in the intensive-care unit during the early post-operative period. A subsequent need to stay in the intensive-care unit was determined by the patient’s condition. Liver function tests and clotting profiles were monitored. Ethical approval was obtained from Eastern Hepatobiliary Surgery Hospital Research Ethics Committee and informed consent was obtained from each patient.
Cell linesCSQT-2 cells (established at the Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, P. R. China), which are a human HCC cell line with high metastatic potential and derived from a PVVT,16 were used for the current study. The Hep3B liver cancer cell lines were obtained from the Shanghai Institutes for Biological Sciences. The cells were cultured in DMEM, and supplemented with 10% foetal bovine serum, 10 units mL-1 of penicillin, and 10 units mL-1of streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
Gene expression profilingThe gene expression profiling of CSQT-2 and Hep3B was completed by Kang Cheng Bio-tech Company. Total RNAs were harvested using TRIzol and the RNeasy Kit (Life Technologies, Grand Island, NY) according to the manufacturer’s instructions, which included a DNase digestion step. After the RNAs were assessed by the Nanodrop ND-1000 and by denaturing gel electrophoresis, the samples were used to synthesise double-stranded cDNA using the Superscript Double-Stranded cDNA Synthesis Kit (Life Technologies, Grand Island, NY). Double-Stranded cDNA was labelled and hybridised to a 12 x 135 K mRNA Expression Microarray in the Nimble Gen Hybridisation System. After hybridisation and washing, the processed slides were scanned with the Axon GenePix 4000B microarray scanner. Raw data were extracted as pair files by NimbleScan software. The P-value was calculated via a paired t-test. The up- and down-regulated gene threshold was set data P-value ≤ 0.05 and a fold change ≥ 2.0.
Assessment of microvessel DensityDuring the clinical research, in order to prove that PVTT contains more microvessels we initially perfused and stained for the PVTT by injecting methylene blue into the hepatic artery during theoperation and performed the CD34 immunohistochemistry of the tissue specimens. Tissue sections of 27 HCC patients (81 samples) with macroscopic PVTT were examined for microvessel density using an immunohistochemical method. The primary antibody was a CD34 monoclonal antibody (Abcam, Cambridge, UK). The numbers of microvessels in 5 highpower fields (magnification × 200) were counted and the mean was calculated. All slides were evaluated by 3 pathologists who were blinded to the nature of the tissue samples.
Real-time PCR and ImmunohistochemistryFresh tissue samples were collected in the operating room and processed within 30 min to minimise RNA and protein degradation. Each fresh sample was transferred in liquid nitrogen and stored at -80 °C until use. Real-time PCR (RT-PCR) was performed to detect SPHK1 gene expression in paired liver samples from 27 Chinese HCC patients. Total RNA was isolated using the TRIzol reagent, and reverse transcription was performed using a one-step RT-PCR kit. The primers of human SPHK1 were 5’-CTTCCTTGAACCATTATGC-3’ (forward primer) and 5’-CCGATACTTCTCACTCTC-3’ (reverse primer). The primers for â-actin were 5’-GTGGGCATGGGTCAGAAG-3’ (forward primer) and 5’-GAGGCGTACAGGGATAGCAC-3’ (reverse primer). The thermal cycle conditions were as follows: 95°C for 30 s followed by 40 cycles of 95 °C for 15 s, 60°Cfor 20 s, and 72 °C for 15 s with a final extension at 72 °C for 10 min. RT-PCR products were visualised by ethidium bromide-stained agarose gels. Immunohistochemical analysis was conducted to study altered protein expression in the same 27 paired liver samples. The sections were incubated with rabbit anti-SPHK1 (1:500, Abcam) overnight at 4°C. Normal goat serum was used as a negative control. After washing, the tissue sections were treated with abiotinylated anti-rabbit secondary antibody (Abcam, Cambridge, UK) followed by further incubation with a streptavidin-horseradish peroxidase complex (Life Technologies, Grand Island, NY). The tissue sections were then immersed in 3, 3’-diaminobenzidine and counterstained with 10% Mayer’s hematoxylin, dehydrated, and mounted.
TMA and immunohistochemical analysisTMAs of the 199 PVTT patients were constructed as described.17 The SPHK1 specific polyclonal antibody was purchased from Abcam. Immunohistochemical staining was performed with the Dako Envision Plus System (Dako, Carpinteria, CA) according to the manufacturer’s instructions. An HCC was considered positive for SPHK1 staining when >10% of the tumor cells demonstrated highly condensed membranous and/or cytoplasmic immunoreactive deposits. The sections were scored using a four-tier scale:
- •
0 = a negative signal (0% - 10%).
- •
1 = a weak signal (10% - 20%).
- •
2 = an intermediate signal (20% - 50%).
- •
3 = a strong signal (> 50%).
The scales 0 and 1 were defined as low, and the scales 2 and 3 were defined as high. All sections were scored independently by two observers who were blinded to the HCC clinicopathological data. The concordance between the scores from different sections of the same tumor was > 90%. All discrepancies in the scoring were reviewed, and a consensus was reached.
Statistical analysisCategorical data were analysed using Fisher’s exact test, and continuous variables were compared using the Mann-Whitney test. Event-time distributions were estimated using the Kaplan-Meier method and were compared using a log-rank test. The Cox regression model was used to perform multivariate analysis to determine the independent factors on survival and recurrence. P < 0.05 was considered statistically significant.
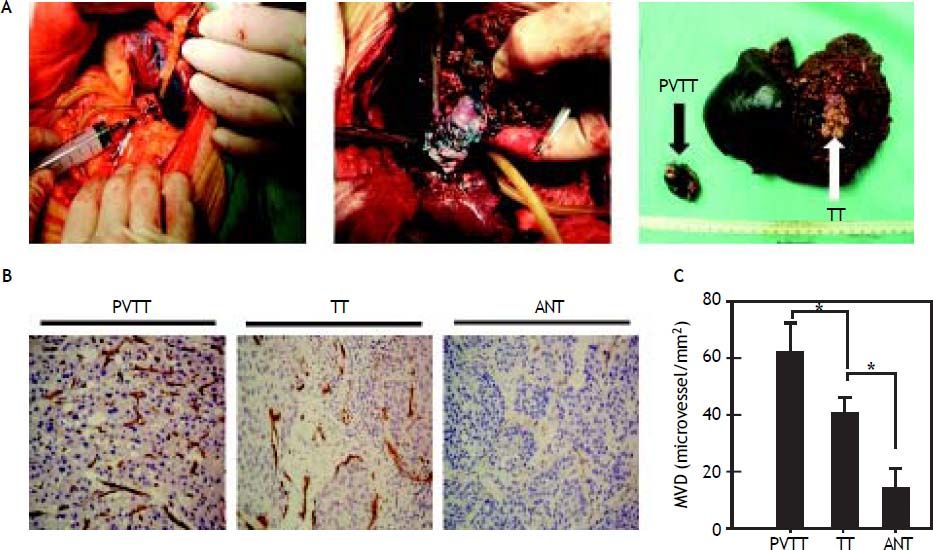
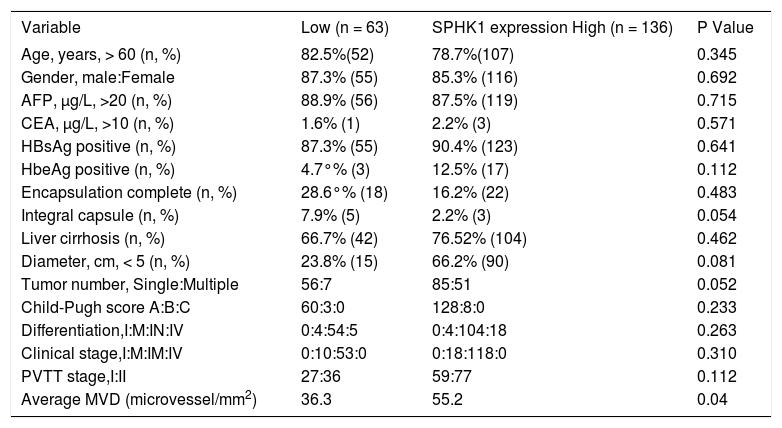
ResultsMicrovessel densityWe see that PVTT tissue was stained by methylene blue during the operation (Figure 1A). We found that the staining intensity in the tumor thrombus was higher compared with the staining intensity in the primary tumor, and the proportion of the stained tumor thrombus was not uniform. The CD34 immunohistochemistry indicated that the microvessel density in PVTT is significantly greater than the microvessel density in the primary tumor and the adjacent non-cancerous tissues (Figures 1B, 1C).
Portal vein tumor thrombus(PVTT)and tumor tissues(TT) were stained using methylene blue during surgery, and the staining intensities were different(arrow) (A). HCC tumor block sections were immunostained for the endothelial marker CD34 (B) to assess the microvessel density in PVTT, the TT, and the adjacent non-cancerous tissues (ANT).The microvessel density in the PVTT and the TT was significantly greater than in the ANT (C).
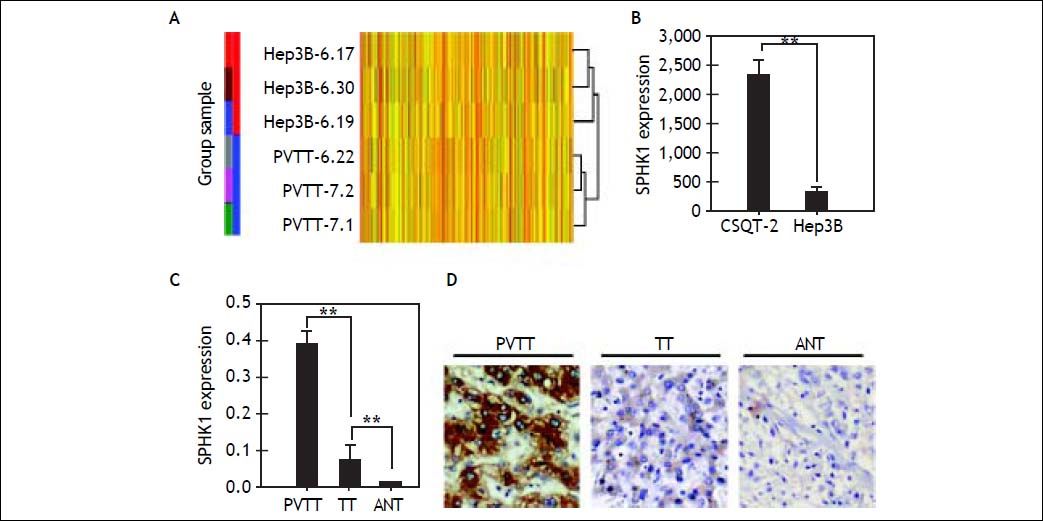
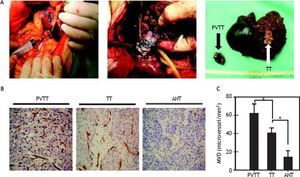
We found that 1,997 out of 6,670 functional genes were differentially up-regulated (Figure 2A). Among all of the up-regulated genes, SPHK1 mRNA expression was elevated most remarkably (up to 8.36-fold) (Figure 2B). The up-regulation of SPHK1 mRNA and protein was further confirmed in 27 HCC patients with PVTT paired tumor and non-tumorous samples by RT-PCR and an immunohistochemical assay (Figure 2C, D). Compared with the non-tumorous samples, we found increased SPHK1 expression in the HCC samples, and 136 of the 199 (68.3%) patients were identified as SPHK1 over-expressors (Score 2-3) (Figure 3D).
Expression of the SPHK1 gene in HCC. The up- regulated genes were examined by gene expression profiling between CSQT-2 cells and Hep3B cells (A). SPHK1 mRNA expression was elevatedmost remarkablyin the gene expression profiling (B). RT-PCR analysis of SPHK1 mRNA expression in PVTT, tumor tissues (TT), and adjacent non-cancerous tissues (ANT) (C). Immunohistochemical analysis of SPHK1 expressions in PVTT, TT, and ANT (D). Hepatocellular carcinoma cells in PVTT and TT were strongly positive for SPHK1 expression in the cytoplasm and on the cell membrane of normal hepatocytes. The ANT showed low detectable SPHK1 expression.
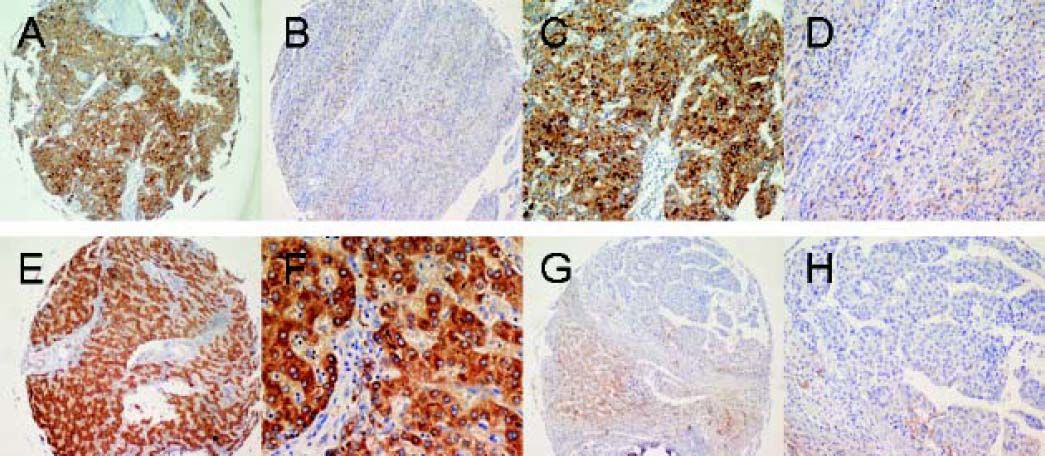
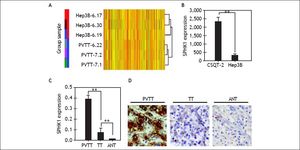
Immunohistochemical analysis of SPHK1 expression in HCCs and the adjacent non-tumorous liver tissues (A-H). Immunohistochemical staining of paired HCC and non-tumoral tissue (A-D). Hepatocellular carcinoma cells in HCC were strongly positive for SPHK1 expression in the cytoplasm and on the cell membrane (A, C). Normal hepatocytes in the non-tumoral tissue showed no detectable SPHK1 expression (B, D). Immunohistochemical staining of another paired HCC and non-tumoral tissue (F, H). HCC cells were strongly positive for SPHK1 expression (E, G). Normal hepatocytes in the non-tumoral tissue showed no detectable SPHK1 expression (F, H). Original magnifications: x 100 (A, B, E, F); x 200 (C, D, G, H).
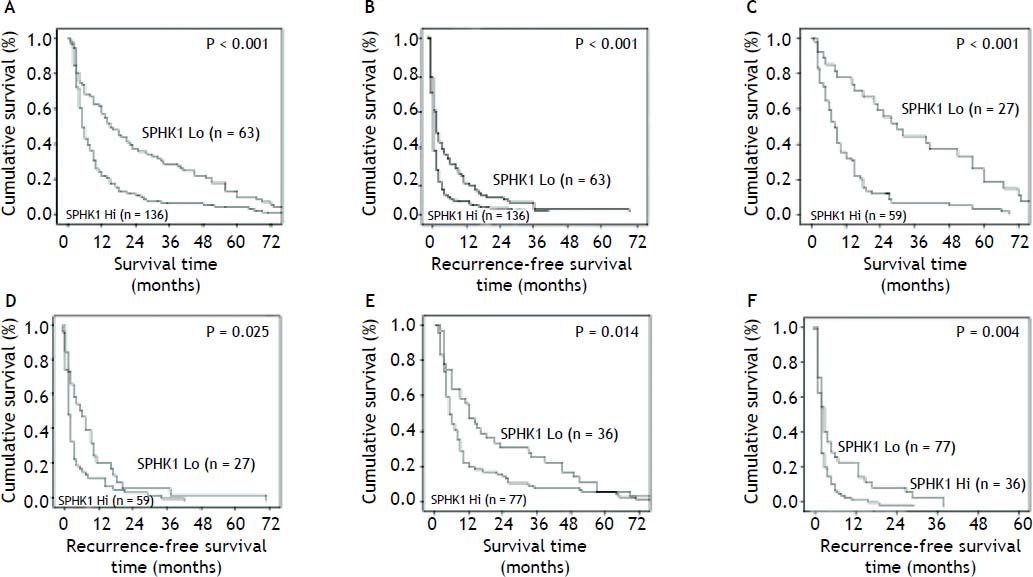
The expression level of SPHK1 was related with worst survival (hazard ratio [HR] 1.799, 95% confidence interval [CI] 1.337-2.368, P < 0.001) and higher recurrence rates (HR 1.451, 95% CI 1.087-1.935, P = 0.011). Kaplan-Meier survival curves with comparisons of SPHK1 over-expression versus SPHK1 under-expression in the 199 HCC patients with PVTT are shown in Figure 4A and 4B. The SPHK1 expression levels were negatively correlated with 1 - and 3- year survival rates (23.5% and 6% for SPHK1 over-expression, respectively, vs. 58.9% and 26% for SPHK1 under-expression, respectively; P < 0.001). The 1- and 3-year cumulative recurrence rates in SPHK1 over-expressing patients were significantly higher than the cumulative recurrence rates in the SPHK1 under-expressing patients (94.5% and 99.4% vs. 79.4% and 95.6%, respectively; P < 0.001).
Clinico-pathological factors and SPHK1 expression level based on immunohistochemistry in TMAs.
| Variable | Low (n = 63) | SPHK1 expression High (n = 136) | P Value |
|---|---|---|---|
| Age, years, > 60 (n, %) | 82.5%(52) | 78.7%(107) | 0.345 |
| Gender, male:Female | 87.3% (55) | 85.3% (116) | 0.692 |
| AFP, μg/L, >20 (n, %) | 88.9% (56) | 87.5% (119) | 0.715 |
| CEA, μg/L, >10 (n, %) | 1.6% (1) | 2.2% (3) | 0.571 |
| HBsAg positive (n, %) | 87.3% (55) | 90.4% (123) | 0.641 |
| HbeAg positive (n, %) | 4.7°% (3) | 12.5% (17) | 0.112 |
| Encapsulation complete (n, %) | 28.6°% (18) | 16.2% (22) | 0.483 |
| Integral capsule (n, %) | 7.9% (5) | 2.2% (3) | 0.054 |
| Liver cirrhosis (n, %) | 66.7% (42) | 76.52% (104) | 0.462 |
| Diameter, cm, < 5 (n, %) | 23.8% (15) | 66.2% (90) | 0.081 |
| Tumor number, Single:Multiple | 56:7 | 85:51 | 0.052 |
| Child-Pugh score A:B:C | 60:3:0 | 128:8:0 | 0.233 |
| Differentiation,I:M:IN:IV | 0:4:54:5 | 0:4:104:18 | 0.263 |
| Clinical stage,I:M:IM:IV | 0:10:53:0 | 0:18:118:0 | 0.310 |
| PVTT stage,I:II | 27:36 | 59:77 | 0.112 |
| Average MVD (microvessel/mm2) | 36.3 | 55.2 | 0.04 |
PVTT: portal vein tumor thrombus. HBsAg: hepatitis B surface antigen. HbeAg: hepatitis B e antigen. CEA: carcino-embryonic antigen. TNM: tumor-node-metastasis. AFP: α-fetoprotein. MVD: microvessel density. † P < 0.05 by χ2 test or Student t test.
Prognostic significance of SPHK1 assessed by Kaplan-Meier analysis and log-rank tests. Kaplan-Meier analysis of the correlation between SPHK1 expression levels and the overall survival or recurrence of 199 HCC patients (A,B). Over-expression of SPHK1 predicts lower overall survival rates (A) and higher cumulative recurrence rates (B). Kaplan-Meier analysis of the overall survival and recurrence in the stage I PVTT patients (C,D), stage II PVTTpatients (E,F). Over-expression of SPHK1 predicts a lower overall survival rate and a higher cumulative recurrence rate than those whose tumors had decreased expression of SPHK1 in the
Kaplan-Meier plots of patients with different stages of PVTT are shown in Figure 4C and 4H. Of the 86 patients at stage I, the 1- and 3-year cumulative recurrence rates were 82.6% and 96.5%, respectively, and the 1- and 3-year survival rates were 48.8% and 18.6%, respectively. Among these patients, 59 were identified as having SPHK1 over-expression and 27 were identified as having SPHK1 under-expression in their tumors. The patients with SPHK1 over-expression had a poorer surgical prognosis in contrast to the patients with SPHK1 under-expression(86.4% and 96.6% vs. 74.1% and 92.6% in 1- and 3-year cumulative recurrence rates, respectively, P = 0.025; 35.6% and 6.8% vs. 77.8% and 44.4% in 1- and 3-year survival rates, respectively, P < 0.001) (Figure 4C and 4D). Of the 113 stage II PVTT patients, the prognosis of the patients with SPHK1 over-expression (n = 77) was also poorer than those with SPHK1 under-expression (n = 36) (1- and 3-year cumulative recurrence rates, 96.1% and 100% vs. 75% and 94.4%, respectively, P = 0.004; and 1- and 3-year survival rates, 22.1% and 7.8% vs. 55.6% and 25%, respectively, P = 0.014) (Figure 4E and F).
DiscussionIn our study, we found that SPHK1 expression was increased at both the mRNA and protein levels in PVTT and HCC tissues and was associated with malignant clinicopathological characteristic. The correlation between SPHK1 expression levels and surgical outcomes was further investigated in a prospective study of 199 HCC patients with PVTT. We showed that both the tumor recurrence and the survival rates differed substantially between patients with over-expression and under-expression of SPHK1 in tumor tissues. The multivariate analysis revealed that SPHK1 expression levels were an independent and significant risk factor affecting recurrence and survival after curative resection. More importantly, SPHK1 over-expression showed enhanced accuracy in predicting the surgical outcome in HCC patients with PVTT. Our data suggests that the over-expression of SPHK1 might be an indicator of a poor outcome in HCC patients with segmentalstage I PVTT- or right and/or left PVTT-stage II.
Our study has provided evidences that up-regulated SPHK1 expression might play an important role in the progression of HCC in patients with PVTT. The up-regulation of SPHK1 in HCC was identified by our study and confirmed by several lines of evidence.18 We found that SPHK1 is increased in PVTT and HCC tissues compared with non-cancerous liver tissues, and a high level of SPHK1 is also expressed in a relatively large number of HCC lesions. It is particularly noteworthy that high SPHK1 expression is associated with a shorter survival time in patients with PVTT. The cumulative 3-year survival rates and recurrence rates were 26% and 95.6%, respectively, in the low SPHK1 expression group, whereas the cumulative 3-year survival rates and recurrence rates were 6% and 99.4%, respectively, in the high SPHK1 expression group.
PVTT was classified to stage C according BCLC staging system and sorafebib was the only selection. But the tumor response of sorafenib was only 2-3% in the SHARP trial.19 Recently, Wang JH, et al.20 have compared several treatment methods in BCLC C patients and surgery acquired the best results. So surgery would be an optional therapeutic strategy to selected PVTT patients, especially stage I-II PVTT which can be radial resected theoretically. And the stage I-II PVTT patients in our study who received hepatectomy also achieved a relatively good survival. According to the PVTT staging system, stage I is considered the early stage of PVTT. Our results suggest that stage I patients whose tumors have an increased expression of SPHK1 could have a shorter time to recurrence and survival than the stage I patients whose tumors had a decreased expression of SPHK1.The analysis of the patients with stage I and II PVTT revealed that patients with SPHK1 over-expression had a poorer surgical prognosis in contrast to the stage I and II patients with SPHK1 under-expression. Therefore, the impact of SPHK1 expression levels on the outcome of these patients could be determined. These results suggest the possibility that SPHK1 can be used as a predictor for patient prognosis and survival.
The limitations of our study were as follows: firstly, all the data of the study was retrospective and secondly, we didn’t include HCC patients without PVTT and we will do this next. The strengths of our study were that we investigated the correlation between SPHK1 expression, tumor recurrence, and survival in HCC patients with PVTT for the first time. And also we validated the function of SPHK1 form basic to clinic. Our results suggest that SPHK1 expression in HCC patients with PVTT is a potential biomarker that has a strong association with disease outcomes and is easy to measure. The over-expression of SPHK1, identified using postoperative immunostaining, might be an early warning sign that HCC patients with PVTT be closely monitored and should receive appropriate adjuvant therapies. Simultaneously, this work might be helpful in providing a potential therapeutic target for PVTT.
Abbreviations- •
ALT: alanine transaminase.
- •
ANT: adjacent non-cancerous tissues.
- •
AST: aspartate aminotransferase.
- •
cDNA: complementary DNA.
- •
CEA: carcino-embryonic antigen.
- •
CIbconfidence interval.
- •
HbeAg: hepatitis B e antigen.
- •
HBsAg: hepatitis B surface antigen.
- •
HCC: hepatocellular carcinoma.
- •
HR: hazard ratio.
- •
mRNA: messenger RNA.
- •
PVTT: portal vein tumor thrombus.
- •
RT-PCR: reverse-transcription polymerase chain reaction.
- •
SPHK1: sphingosine kinase 1.
- •
TMA: tissue microarray.
- •
TT: tumor tissues.
We thank the patients and clinicians at the VI Department of Hepatic Surgery of EHBH, Second Military Medical University, for their participation in the study. There are no conflicts of interest to disclose.
Grant SupportThis work was supported China National Funds for Distinguished Young Scientists (Grant No: 81125018). National Natural Science Foundation of China (Grant No: 81101831, NO: 81101511) Youth science funds of The Second Military Medical University (Grant No: 2012QN21).
FundingThis work was supported China National Funds for Distinguished Young Scientists (Grant No: 81125018). National Natural Science Foundation of China (Grant No: 81101831, NO: 81101511); Youth science funds of The Second Military Medical University (Grant No: 2012QN21).
Ethical ApprovalNot needed.
ContributorsCSQ proposed the study. SJ, HYY, and SJX wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. CSQ is the guarantor.
Competing InterestNo benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.