Background. Transarterial chemoembolisation (TACE), having demonstrated survival benefits, is the treatment of choice in intermediate-stage hepatocellular carcinoma, although there is great heterogeneity in its clinical application.
Material and methods. A survey was sent to the Madrid Regional hospitals to assess applicability, indications and treatment protocols. The assessment was made overall and according to the type of hospital (groups A vs. B and C).
Results. Seventeen out of 22 hospitals responded (8/8 group A, 9/ 14 group B-C). All do/indicate transarterial chemoembolisation, 13/17 at their own facilities. Eight of the 17 hospitals have multidisciplinary groups (5/8 A, 3/9 B-C). Nine hospitals perform > 20 procedures/year (7 group A), and 6 from group B-C request/perform < 10/year. It is performed on an “on-demand” basis in 12/17. In 5 hospitals, all the procedures use drug-eluting beads loaded with doxorubicin. The average number of procedures per patient is 2. The mean time from diagnosis of hepatocellular carcinoma to transarterial chemoembolisation is ≤ 2 months in 16 hospitals. In 11/17 hospitals, response is assessed by computed tomography. Radiological response is measured without specific criteria in 12/17 and the other five hospitals (4 group A) assessed using standardised criteria.
Conclusion. Uniformity among the Madrid Regional hospitals was found in the indication and treatment regimen. The use of DEB-TACE has become the preferred form of TACE in clinical practice. The differentiating factors for the more specialised hospitals are a larger volume of procedures, decision-making by multidisciplinary committees and assessment of radiological response more likely to be standardised.
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. The Barcelona Clinic Liver Cancer (BCLC) staging system is currently the most widely-accepted for determining prognosis.1–3 This system not only provides a prognosis, but it also determines the optimal treatment strategy for each stage. In intermediate-stage HCC (BCLC B), which includes patients who are not candidates for curative treatments due to tumour extension, who have no vascular invasion or extrahepatic spread, no tumour-related systemic symptoms and preserved liver function (Child-Pugh class A or B), the recommended treatment is transarterial chemoembolisation (TACE).4,5 This recommendation is based on a meta-analysis which included controlled studies and showed that TACE significantly increased survival in patients with unresectable HCC.6
However, there is a great deal of heterogeneity among intermediate-stage patients given the large variability in tumour burden and in terms of liver function, which can range from compensated cirrhosis to manifestations such as ascites or hyperbilirubinaemia. This means that in practice, TACE is not always indicated in these patients.7,8
Moreover, the TACE procedure itself is highly variable and there is no consensus on how it should be applied. It may be performed according to a fixed schedule or on demand, depending on the response or the appearance of new tumour nodules.9 The chemotherapeutic agent infused with lipiodol varies, as does the embolic agent, and delivery may be anywhere from super-selective to lobar. The procedure is not without complications, which can be severe, such as decompensation and deterioration of liver function, or in relation to ischaemic events in the non-tumour tissues.8,10,11 In recent years there have been major technical modifications such as the introduction of charged polyvinyl alcohol beads (drug-eluting beads-DEB) with doxorubicin (DEBDOX), use of which has become widespread in clinical practice.12 A phase II study demonstrated that TACE with these particles had a better side-effect profile, although the increase in efficacy was not statistically significant with respect to conventional TACE with lipiodol.13
In view of this possible great variability in the practical application of TACE as treatment for BCLC-B HCC, a survey was designed and sent to the physicians responsible for this disease in the different gastroenterology departments of hospitals in Comunidad Autónoma de Madrid (CAM) [Madrid Region] to ask them about their actual practice.
Material and MethodsAn annual conference is held in CAM on the diagnosis and treatment of HCC. It is organised by gastroenterology specialists with a special interest in hepatology and this complication of liver disease in particular. Prior to the 2012 conference, a survey was sent to physicians specifically responsible for the care and treatment of HCC in the gastroenterology departments of the different hospitals in the CAM designed to assess whether there were differences in the practical application of TACE in the treatment of BCLC-B HCC.
CAM is a Spanish region at the centre of country. Its capital is the city of Madrid, which is also the national capital of Spain. It has a population over 6 million people. The survey was sent to 22 Madrid hospitals with gastroenterology departments or units and responses received from 17. The hospitals were divided according to type into level A (large hospitals providing complex care with all the medical specialist areas and which are generally regional or national referral centres for different diseases) vs. level B (medium-sized hospitals which deal with all types of disease but do not perform transplants or cover specialist areas only provided in the referral hospitals) and level C (small hospitals which do not provide complex care).
The survey consisted of 22 questions, each with 4 options, with the aim of unifying the answers, and the person completing it had to choose the option that best conformed to their usual clinical practice. The questions referred to different aspects of the application of TACE at each hospital and gastroenterology department: structure and care provision, indication, technical aspects and monitoring of the treatment response. Gastroenterologists were the main target for the survey. However those questions referred to the TACE technical aspects were obtained from Interventional Radiologists at each site, either in the multidisciplinary teams or from direct contact with refernce radiologists. The survey is shown in appendix 1.
For each question, the results are expressed as percentages for all the hospitals overall and by type of hospital according to degree of specialisation.
ResultsStructure of the units and care provisionResponses were obtained from 17 of the 22 hospitals surveyed (8/8 from group A, 9/14 group B-C). In terms of availability of TACE, 13 hospitals have the technique available on site and 4 from group B-C have a referral hospital to which they can refer their patients. As shown in figure 1, 9 hospitals perform more than 20 procedures a year, 7 of these being from group A, and 6 hospitals from group B-C perform or indicate fewer than 10 procedures a year.
The composition of HCC treatment units, and how they deal with therapeutic decision-making, was evaluated. In 8 hospitals (47.1%), decisions are made by multidisciplinary committees and in 2 hospitals, by a committee composed of physicians from the Gastroenterology and Radiology Departments. In the rest of the hospitals, decisions are made within the department concerned or individually by the doctor responsible for the patient. Multidisciplinary committee decision-making takes place at 5/8 hospitals in group A (62.5%) and at 3/9 in group B-C (33.3%).
Indication for TACEThe indication for TACE is governed in all the hospitals by BCLC intermediate-stage diagnostic criteria, and one hospital gives more priority to absence of technical contraindication than actual BCLC stage.
If the patient has an adverse profile for application of TACE, in 12 hospitals (70.6%), this is assessed on an individual basis; in 1 hospital, TACE is always applied as first choice; in 2 hospitals, TACE is performed in patients with Child-Pugh score > 7 only if the procedure can be selective and in 1 hospital, alternative first-line techniques are assessed.
Technical aspects of TACE applicationDEBDOX-based TACE is the most commonly used in CAM. It is the standard in 5 hospitals and the preferred in another 7, while conventional TACE with lipiodol is the standard in only 1 hospital and the preferred in another 4 (DEBDOX vs. conventional TACE, 70.6 vs. 29.4% respectively). When asked about the most-used DEBDOX particle size, 5 hospitals did not respond and in 3, it depended on the case. Of the other 9 hospitals, 4 answered 100-300 μm, 3 stated 300-500 μm and 2 answered 500-700 μm.
The treatment regimen is “on demand” in 12 hospitals or “mixed” (initially more than one session pre-arranged as per protocol until complete response is obtained and then on demand) in the other 5 hospitals (3/8 from group A and 2/7 from group B-C).
The most common average number of procedures per patient is 2 (9 hospitals; 52.9%), with only 1 hospital, from group B-C, having an average over 3 (Figure 2).
The TACE aplication in patients with large hepatic tumour extension was evaluated. First, bilobar multicentric tumour, the procedure is performed over two sessions, on one lobe per session, in 10 hospitals (58.8%), and second, in HCC with a nodule larger than 5 cm, in 13 hospitals (76.4%), they assess the possibility of mixed treatment, radiofrecuency + TACE, in the same session or sequential if response is not complete after TACE (7/8 group A, 6/ 9 group B-C).
Monitoring of response to TACETo find out how the Units work, they were asked about the time interval between staging and performing of TACE; this was 1 month in 12 hospitals (6/8 hospitals from group A, 6/9 group B-C) and 2 months in another 4 hospitals. Only 1 hospital from group A reported a delay that can be as long as 3 months.
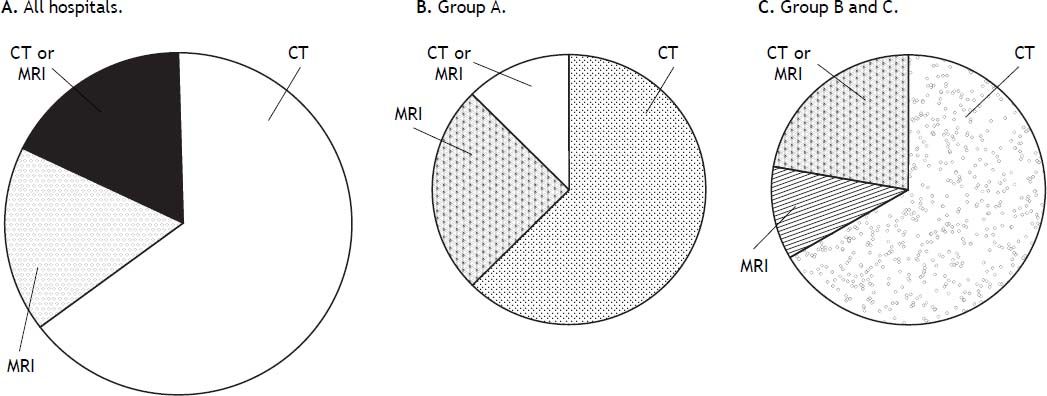
In terms of how long after the technique the follow-up imaging tests are performed, the average time was 4 weeks in 10 hospitals (58.8%; 5/8 group A, 5/9 group B-C), 6 weeks in 6 hospitals and 8 weeks in 1 hospital. As is shown in figure 3 computed tomography (CT) is the preferred dynamic imaging technique and is the one most commonly used in 11 hospitals (64.7%) distributed evenly across hospital groups (5/ 8 group A, 6/9 group B-C). In the other hospitals, 3 use magnetic resonance imaging (MRI) as first choice, and the other 3, CT or MRI indifferently.
Response was evaluated by standardised criteria (RECIST or mRECIST) in 5 hospitals (29.4%), 4 of these 5 from group A, and in the other 12, at the discretion of the radiologist.
From the point of view of therapeutic failure with the technique, in 13 hospitals (76.5%; 5/8 group A, 8/ 9 group B-C) it is considered if complete response is not obtained after 2 sessions; in 2 hospitals after 3 sessions; in 1 hospital after multiple procedures and in 1, it is not defined. The appearance of new lesions, no response after various sessions and technical complications preventing further sessions are considered to signify therapeutic failure in 12 hospitals (70.6%). In the rest of hospitals, it is defined primarily by lack of response in 1 and by appearance of new lesions in the other 4; none of the hospitals considers technical contraindication after a previous TACE session on its own to determine therapeutic failure.
With respect to treatment given after therapeutic failure, the first-line option in all the hospitals is treatment with sorafenib. They were asked if any of the hospitals used sorafenib as adjuvant treatment with TACE; in 3 hospitals, it is assessed as a possibility on an individual basis and in 9, they have used it on occasion.
After achieving complete response, patients are followed up with radiological tests every 3 months in 7 hospitals and every 3 months for the first year approximately then every 6 months in the other 10 hospitals.
DiscussionHCC is the most common form of primary liver cancer and generally occurs in patients with underlying liver disease, in our setting mainly cirrhosis. This type of tumour cannot be treated with radiotherapy or cytotoxic drugs due to lack of response or toxicity and it is therefore generally diagnosed and treated in Gastroenterology Departments. In view of the fact that TACE is the treatment of choice recommended by current clinical guidelines in intermediate-stage (BCLC-B) HCC, the aim of this survey was to determine how TACE is actually used in our setting for BCLC-B HCC by assessing indications, treatment and evaluation of response.1–3
Although it would seem to be a clearly-defined clinical situation, in actual clinical practice, it could be an extremely heterogeneous group. It was therefore decided that it would be of clinical interest to find out more about how it is applied in clinical practice by the gastroenterology departments in CAM. This statement is based on the fact that this group has been observed to include patients with both compensated and decompensated liver function, while in the latter case, treatment with TACE is contraindicated.2 Additionally, the larger the tumour mass, the lower the likelihood of achieving a complete response with TACE or this could even be a relative contraindication (nodule ≥ 10 cm).7,8 As a result, other therapies such as Yttrium-90 microsphere radioembolisation, or the combination of TACE with other locoregional treatments such as radiofrequency, or systemic with sorafenib, are being assessed in ongoing clinical trials with the aim of resolving these problems and improving the classification of and therapeutic options for these patients.2,7,11
In turn, if evaluated from the viewpoint of the recommended treatment, TACE, we note that there is no standardisation of the procedure and therefore comparison is difficult in clinical practice. If we look at the standard or classic TACE with lipiodol as carrier for the chemotherapeutic agent, generally doxorubicin, there can be a high degree of variability in terms of: application in super-selective mode vs. lobar embolisation or non-selective procedure; the embolising agent used after the infusion of lipiodol emulsified with the chemotherapeutic agent; or whether the regimen for the procedure is on demand, fixed or mixed. It is true that there is evidence of greater response in selective treatments vs. lobar and in TACE compared to transarterial tumour embolisation without chemotherapy, but it has not been demonstrated that an on-demand treatment regimen is inferior to a fixed regimen.6–9 For example, the regimen most used in the CAM hospitals is TACE on-demand, and similar results are seen among the hospitals in terms of how they proceed in intermediate-stage cases with unfavourable profile for TACE.
In view of the above, and having defined the clinical situation, this survey was sent around CAM to determine how TACE is indicated and performed in the patient with intermediate-stage HCC. Even though CAM has a range of both high-complexity-care hospitals, leaders in their class in Spain, and other small hospitals, and logical differences were detected (greater number of procedures, working in multidisciplinary units and using standardised protocols for radiological response), no differences were observed in the indication, the technique used and performing of the procedure, or in the evaluation of radiological response to the therapy. This speaks in favour of the high level of training and care provision at all the hospitals that responded to the survey. Therefore this type of patient within the HCC setting is adequately addressed regardless of the hospital group, which can be explained given the close relationship between gastroenterology specialists, which is improved by specific meetings such as the ones performed periodically in the Madrid HCC group.
One point specifically addressed in the survey due to its application in clinical practice was the use of a new form of TACE called DEB-TACE. In this procedure, polyvinyl alcohol spheres of different diameters (100 to 700 μm) loaded with doxorubicin are selectively infused into the tumour nodule, combining the effect of more efficient and sustained distribution of the chemotherapeutic agent with lower systemic exposure, with the embolising effect that causes ischaemia/hypoxia of the tumour tissue.14 PRECISION V was a phase II study with the objective of comparing the efficacy and safety of DEB-TACE (DEBDOX) with those of conventional TACE, as standard treatment in BCLC stage A or B patients not candidates for treatment by surgical resection or percutaneous ablation. Although overall, no significant differences were found in the objective response or stabilised disease rates, there was a tendency towards better disease control with less toxicity, in addition to being a procedure with levels of consistency and reproducibility superior to conventional TACE.13 After a rigorous review of the literature and based on their personal experience, a group of experts recently sought to reach a consensus on recommendations regarding indications and contraindications, technical aspects of the angiographic procedure and treatment schedule.12 In accordance with these practice guidelines, a study was recently published showing that the use of DEB-TACE in clinical practice, applying strict technical and selection criteria, achieves the best survival outcomes ever obtained.15 Use of this new technique for application of TACE has quickly spread to become routine clinical practice in CAM hospitals, to the extent that it has largely replaced conventional TACE; it is now the only technique used or the most predominant in 12 of the 17 hospitals that responded to the survey (70.6%). These results are in concordance with the evolution of this treatment for HCC. If we take a look of what is done in other countries, we can observe in a survey of locorregional treatments for HCC from the Italian Society of Interventional Radiology that 52% of procedures of TACE are performed with DEB-TACE and 80.8% of centres use DEB-TACE with DC Bead®.16 In another survey from the Society of Interventional Radiology in the United States DEB-TACE is also the preferred intraarterial therapy for HCC.10
The number of procedures depends on many factors, including whether the TACE regimen is fixed or on demand according to radiological response, and whether liver function parameters allow the procedure to be repeated. There is evidence that one single procedure is not enough to prolong survival in the patient with intermediate-stage HCC, but what we still do not know is whether a fixed TACE regimen is better than application on demand according to response, which may be better tolerated and perhaps preferable with conventional TACE.8,9 It is important to point out that in the PRECISION V study, DEB-TACE was applied according to a fixed regimen but DEB-TACE regimen on demand is the preferred in the CAM hospitals and also in the Italian survey.16 In clinical practice, the usual would be repeated application over time, but we found that the most common is 2 or 3 sessions of TACE per patient, consistent with recently published recommendations. Moreover, application is mainly according to an on-demand regimen and is consistent with the intention to treat results obtained in a retrospective study of an Italian cohort with on-demand application of TACE, in which only 22 out of 151 patients, approximately 15%, had a third session.9 A new scoring system, the “ART score”, designed to determine which patients will benefit most from a second TACE procedure, may be of help in this context.17 Another point is the performance of mixed treatments as TACE plus sorafenib or radiofrecuency that is reported in this survey as in the Italian and American surveys.10,16 This kind of treatment is not recognized in the BCLC system but is currently evaluated as a potential treatment and used in clinical practice that could decrease the number of procedures applied to obtain response and also to improve the results.
One important aspect is how we define response and when to stop the TACE treatment. The response is evaluated using dynamic radiological imaging tests, with no solid evidence in favour of CT over MRI or viceversa.18,19 Despite the fact that in conventional TACE, the lipiodol can interfere with evaluation of response by CT scanning,18 as found in the CAM hospitals, CT still tends to be the technique of choice, although this may be related in part to the more general availability of CT compared to MRI in these centres.
Once the dynamic imaging test has been performed, it is important to apply standardised criteria to provide consistency in the evaluation of response. For tumours such as in HCC in which locoregional treatments are applied, the measurement of “viable tumour tissue” according to arterial uptake of contrasts in dynamic imaging tests provides a more realistic assessment of the response than simply the change in tumour size (RECIST criteria). For that very reason, a group of experts brought together by the American Association for the Study of Liver Diseases (AASLD) designed and recommended the application of the viable tumour criteria for clinical studies in HCC, leading to the modified RECIST criteria (mRECIST).20 This evaluation of response is the one recommended in the EASL-EORTC clinical practice guidelines for the design of clinical studies,2 but its systematic use is not recommended in clinical practice, as it requires a more comprehensive radiology report and longer working time per test for the radiologist. These factors are obvious limitations in terms of its inclusion in routine clinical practice, as made evident by the results of our survey especially in medium and small CAM centres.
Lastly, another important aspect is to define treatment failure and therefore when to discontinue treatment with TACE. A published algorithm recommended by a group of experts and later defined in a clearer more practical form by the team at Hospital Clinic de Barcelona are well described in references.8,19 When treatment failure or untreatable progression occur, the recommended treatment for the next stage, sorafenib, should be given. As expected, these recommendations are widely followed in CAM, in over 70% of the hospitals, with no measurable difference between hospital types, and there is consensus throughout on the indication of sorafenib as treatment of choice in this clinical situation. In addition, there is application of the most recent clinical guidelines on radiological monitoring with complete response, as recommended in the EASLEORTC clinical practice guidelines; every 3 months the first year then every 6 months by CT or MRI.2
Another point is the combination of DEB-TACE and sorafenib, in intermediate or advanced stage HCC, to improve results based in the rationale of inhibition of proangiogenic factors promoted by hypoxia. This combination is increasingly used in clinical practice as was reported by some centres of CAM, but the current evidence is still controversial. This combination treatment is likely to improve survival but could be associated with a significantly increased risk of adverse reactions. Clinical trials are ongoing in intermediate-stage HCC patients to resolve this question.21–23
There are several important limitations to this investigation. First, the survey was done in a small size sample of centres, although all the centres of the CAM region were asked to participate and most of the, 17 out of 22, centres collaborated in the study. Second, the survey was done by gastroenterology specialists so certain items related with TACE technique might not reflect clinical practice as we would like. However, at each centre the physicians who filled out the questionnaire were the responsible for HCC at these centres and they work closely with the interventional radiologists directly or in multidisciplinary committees. Furthermore, with the difficult purpose to decrease variability in clinical practice, concise questions were designed for this and responses were limited to 4 options, assuming the risk that probably some data have not been captured. Lastly, survey data are always limited by reporter bias or response bias, but the good relationship between physicians, that are collaborators of we called the “Madrid Hepatocellular Carcinoma Group”, could ensure that responses reflected true practices.
ConclusionDespite the heterogeneity both among BCLC-B patients and in the application of the treatment of choice, TACE, there is homogeneity in the indication, treatment regime and evaluation of response testing among CAM hospitals, which suggests a high level of adherence to the recommendations in the current clinical guidelines in clinical practice. The use of DEB-TACE has become the preferred form of TACE in clinical practice. The differentiating factors for the more specialised hospitals are a larger volume of procedures, decision-making by multidisciplinary committees and assessment of radiological response more likely to be standardised.
Abbreviations- •
BCLC: Barcelona Clinic Liver Cancer.
- •
CAM: comunidad de Madrid [Madrid region].
- •
CT: computered tomography.
- •
DEBDOX: drug-eluting beads with doxorubicin.
- •
HCC: hepatocellular carcinoma.
- •
MRI: magnetic resonance imaging.
- •
TACE: transarterial chemoembolisation.
The authors declare that there are no conflicts of interest.
FundingSince its inception in 2008, the logistical aspects of the “Madrid Hepatocellular Carcinoma Group” annual conference on diagnosis and treatment of hepatocellular carcinoma have been funded by Bayer. Bayer, however, did not participate in the design, the analysis of the responses, the drafting of or the conclusions derived from the survey.
APPENDIX 1.
| SURVEY |
| Question 1: Can chemoembolisation be ordered/performed in patients with HCC at your hospital? |
| A) Yes, it is done in the same hospital. |
| B) Yes, but it is requested at the specialist referral hospital. |
| C) The simple cases are done in the hospital and the complex cases are referred to the specialist referral hospital. |
| D) No. |
| Question 2: In which intermediate-stage patients is chemoembolisation indicated at your hospital? |
| A) In all. |
| B) Only if liver function is good, PS 0-2 and vascular invasion or extrahepatic spread are ruled out. |
| C) Only when there is no contraindication to the procedure. |
| D) Only patients with the most favourable profiles. |
| Question 3: In intermediate-stage patients with adverse profile: |
| A) We always indicate chemoembolisation and continue or not according to response. |
| B) If they have moderately impaired liver function (Child-Pugh class B > 7), we indicate chemoembolisation but in very selective procedures. |
| C) We go straight to another treatment option. |
| D) We make individual decisions in each case. |
| Question 4: Approximately how many chemoembolisation procedures for HCC do you perform/request in a year? |
| A) 0-10. |
| B) 11-20. |
| C) 21-50. |
| D) > 51. |
| Question 5: What type of chemoembolisation is performed in your hospital in patients with HCC? |
| A) TACE DC Beads. |
| B) Conventional chemoembolisation with lipiodol. |
| C) Both, mainly DC Beads. |
| D) Both, mainly conventional chemoembolisation. |
| Question 6: What percentage of all chemoembolisations for HCC performed at your hospital are TACE with DC Beads? |
| A) < 25%. |
| B) 25-50%. |
| C) 51-75%. |
| D) 100%. |
| Question 7: In hospitals where both techniques are used, the selection of one procedure over another is based on which of the following: |
| A) Economic criteria. |
| B) Characteristics of the HCC (single lesions, bilobar or multicentric involvement). |
| C) Radical nature of the treatment (definitive treatment vs. bridge to transplantation). |
| D) Purely angiographic criteria. |
| Question 8: Do you follow a set treatment regimen when treating with TACE? |
| A) Fixed. |
| B) On demand, according to radiological response. |
| C) Mixed, initially fixed and if a response is achieved, on demand if progression occurs. |
| D) No set regimen. |
| Question 9: What DC Beads particle size is generally used at your hospital? |
| A) 100-300. |
| B) 300-500. |
| C) 500-700. |
| D) Depends on the patient. |
| Question 10: What is the average number of procedures performed per patient for whom TACE is indicated as treatment for their HCC? |
| A) 1 |
| B) 2 |
| C) 3 |
| D) > 3 |
| Question 11: What is the average interval between tumour staging (CT or MRI) and TACE? |
| A) 1 month. |
| B) 2 months. |
| C) 3 months. |
| D) Generally more than 3 months. |
| Question 12: When the patient has bilobar multicentric HCC: |
| A) I treat both lobes in the same session. |
| B) I treat each lobe in separate sessions 2 months apart. |
| C) I never prescribe chemoembolisation with that tumour burden. |
| D) I do mixed treatment with radiofrequency pre-TACE or post-TACE. |
| Question 13: In cases of large tumours (between 5 and 10 cm): |
| A) I do mixed treatment (radiofrequency and TACE). |
| B) I do TACE, and I only consider radiofrequency in the case of partial efficacy. |
| C) I only do TACE until complete necrosis. |
| D) I prescribe other treatments. |
| Question 14: How long after the TACE do you perform the dynamic radiological imaging test to assess the response to TACE? |
| A) 4 weeks. |
| B) 6 weeks. |
| C) 8 weeks. |
| D) > 8 weeks. |
| Question 15: Which dynamic imaging test do you most often use to assess the efficacy of TACE? |
| A) Abdominal CT. |
| B) Liver MRI. |
| C) Either CT or MRI. |
| D) Contrast-enhanced ultrasound. |
| Question 16: At your hospital, the radiology department reports the result of the evaluation of response to chemoembolisation according to: |
| A) RECIST criteria. |
| b) Modified RECIST criteria. |
| C) The radiologist’s subjective criteria. |
| D) Objective non- RECIST criteria with expert radiologist. |
| Question 17: How many sessions do you consider necessary to assume therapeutic failure of TACE at your hospital? |
| A) 1. |
| B) 2. |
| C) 3. |
| D) > 3. |
| Question 18: What do you consider to be failure of the treatment with chemoembolisation? |
| A) Lack of complete response after various sessions. |
| B) Appearance of new lesions or growth of those treated. |
| C) Complication associated with the technique that contraindicates a new procedure. |
| D) All the above. |
| Question 19: What treatment do you generally indicate after therapeutic failure to the treatment with chemoembolisation in HCC? |
| A) Radiofrequency. |
| B) Sorafenib. |
| C) Radioembolisation with Yttrium-90. |
| D) Other. |
| Question 20: Do you give adjuvant therapy with sorafenib in patients on treatment with chemoembolisation? |
| A) Never. |
| B) Occasionally. |
| C) Regularly. |
| D) Only in patients in advanced stages with predominantly liver disease on treatment with sorafenib. |
| Question 21: In patients with complete response, how often do you perform the radiological monitoring? |
| A) Every 3 months. |
| B) Every 6 months. |
| C) Every 3 months in the first year, then every 6 months. |
| D) No set schedule. |
| Question 22: How are therapeutic decisions made for patients with HCC at your hospital? |
| A) Individual - physician responsible. |
| B) HCC committee in your unit. |
| C) HCC committee made up of radiologists and hepatologists. |
| D) Multidisciplinary Committee. |