For long, bleeding in cirrhotic patients has been associated with acquired coagulation disorders. The aim of our study was to investigate the impact of acquired coagulation disorders on bleeding risk in cirrhotic patients.
Materials and methodsBlood samples were collected from 51 cirrhotic patients with (H+) or without (H−) bleeding events and 50 controls matched by age and sex. Thrombin generation was assessed as endogenous thrombin potential (ETP). Hemostatic balance was assessed by means of ratios of pro- to anticoagulant factors and by ETP ratio with/without protein C (PC) activator (ETP ratio).
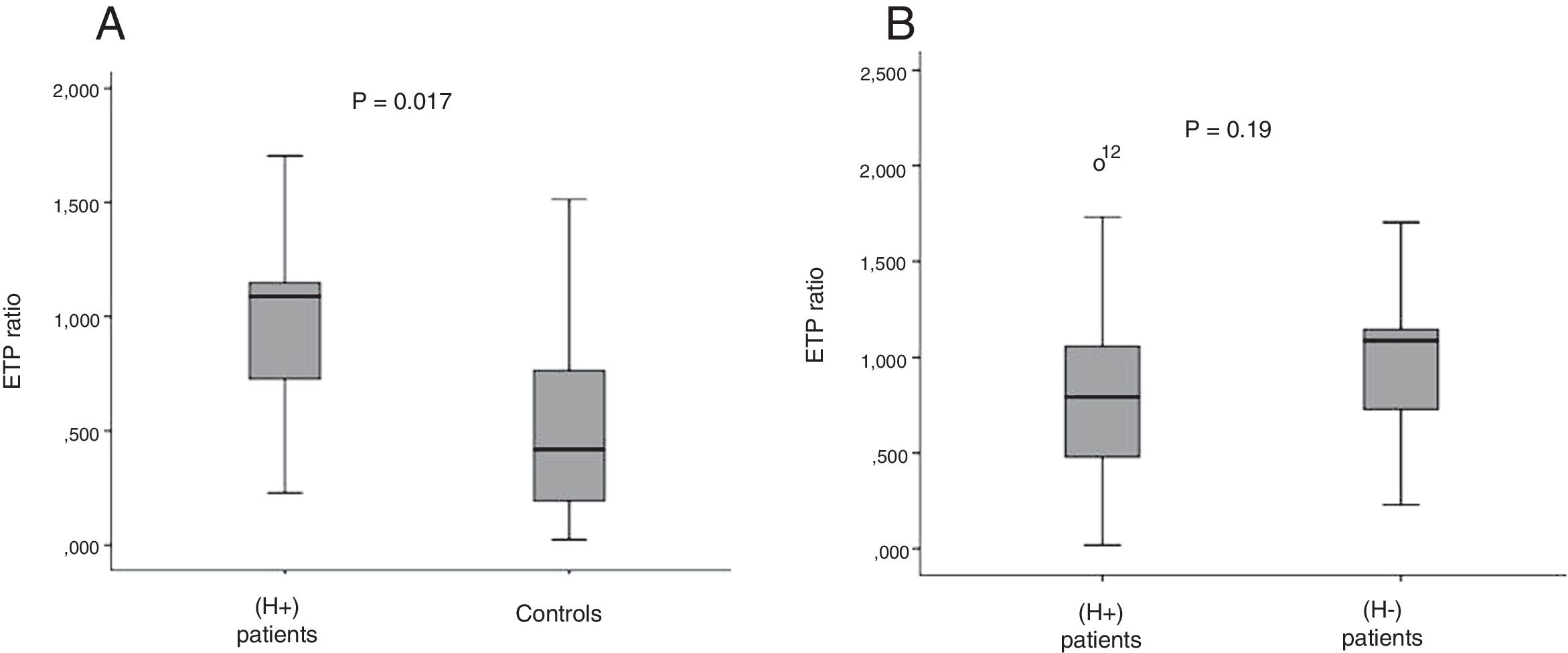
ResultsBleeding events occurred in 9 patients (17.6%). Compared with controls, VIII/anticoagulant factors, VII/PC and XII/PC were significantly higher in (H+) patients. No significant difference as regards all ratios across patient groups was detected. ETP ratio was significantly higher in (H+) patients than in controls (p=0.017). However, there was no significant difference between patient groups as regards ETP ratio.
ConclusionHemostatic balance is shifted toward a hypercoagulability state even in cirrhotic patients who experienced bleeding. These findings provide evidence against traditional concept of hemostasis-related bleeding tendency in cirrhotic patients.
Bleeding often complicates the clinical course of cirrhosis. The most frequent and potentially life-threatening site of bleeding is the gastrointestinal tract. It could result from a variety of lesions including gastro-esophageal varices and portal hypertensive gastropathy or other lesions seen in the general population like esophagitis, Mallory-Weiss tears and peptic ulcer disease. Mortality rates related to gastrointestinal bleeding are high; up to 30% of initial variceal bleeding episodes are fatal [1]. Patients with cirrhosis may also experience cutaneous or mucosa-associated bleeding including bruising, purpura, epistaxis, gingival bleeding, menorrhagia, and bleeding associated with invasive procedures such as liver biopsy. For long, this increased hemorrhagic risk has been thought to be related to acquired hemostatic disorders in cirrhotic patients. In fact, liver synthesizes almost all coagulation factors with the exception of factor VIII. It has been thought that the reduced synthesis of clotting factors was responsible for abnormal conventional laboratory tests exploring global clotting activation, such as prothrombin time (PT) and activated partial thromboplastin (aPTT). However it has been noted that despite prolonged PT and aPTT, many cirrhotic patients do not experience bleeding even after liver biopsy or other potentially hemorrhagic procedures [2]. Furthermore, some cirrhotic patients do experience thromboembolic events despite abnormal conventional laboratory tests. Thus far, only few studies have investigated the impact of coagulation disorders on bleeding risk in cirrhotic patients [3,4].
This study aims to assess the relationship between hemostatic disorders and the risk of bleeding in cirrhotic patients through investigating hemostatic balance in cirrhotic patients with and without bleeding in comparison with controls.
2Materials and methodsThe study was performed in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the local ethics committee. All patients gave their informed consent prior to inclusion in the study.
2.1Patient population and study designFifty-one cirrhotic patients constituted the group of cases and 50 healthy controls matched for age and sex were included in a cross-sectional study. Data regarding bleeding episodes, characteristics of cirrhosis and medication were abstracted from medical records. The severity of cirrhosis was estimated according to Child Turcotte Pugh score. Patients who had bleeding episodes within 2 months before or after inclusion were identified. For each of these patients, site and severity of bleeding were determined. Patients who experienced bleeding with deglobulisation (fall of the hemoglobin of 2g/dl or more) requiring or not a transfusion were considered as having severe hemorrhage. Exclusion criteria were hepatocellular carcinoma or any other malignancy, bacterial infection, chronic inflammatory disease, nephrotic syndrome, renal failure, known inherited coagulation abnormalities and use of anticoagulant or anti-platelet drugs. Patients were divided into two groups: (H+) group included patients who experienced bleeding episodes and (H−) group included patients who did not.
2.2Blood collectionBlood sample was drawn through venipuncture into plastic tubes containing 3.2% sodium citrate as an anticoagulant in the proportion of one to nine parts of anticoagulant/blood. Poor platelet plasma (PPP) was obtained after a double blood centrifugation at 2500×g for 15min. PPP was aliquoted and frozen at −80°C until coagulation tests were performed.
2.3Laboratory tests2.3.1Conventional laboratory testsThe prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured, respectively, with the human thromboplastin (neoplastin, STAGO) and the automated APTT reagent (APTT, STAGO). The fibrinogen was measured with (STA fibrinogen, STAGO).
2.3.2Pro and anticoagulant factorsThe pro and anticoagulant factor activities were determined as functional activities using STAGO reagents: Factor VIII and XII activities were measured with standard aPTT based clotting assays (respectively with DEFICIENT VIII and IMMUNODEF XII). Factor VII, factor V and factor II activities were measured with PT based clotting assays (respectively with DEFICIENT VII, DEFICIENT V, IMMUNODEF II). Protein C (PC) and Protein S (PS) activities were measured using clotting assays (STACLOT PC and STACLOT PS). Antithrombin (AT) activity was determined using chromogenic assay (STACHROM AT). All tests were performed on STA COMPACT MAX (STAGO; Asnières France). Platelet count was performed on the hematology analyzer (SYSMEX XT 2000i; Cobe, Japan).
2.3.3Thrombin generationThrombin generation (TG) was measured by means of calibrated automated thrombin method (thrombiniscope-Thermosystem). The coagulation was triggered in the PPP after addition of tissue factor 1pmol/L and phospholipids (PPP reagent low-STAGO) into two steps: in the absence then in the presence of protein C activator (PROTAC; STACLOT PC STAGO). Registration of the thrombin generated over time was carried out with a fluorogenic substrate (FLUCAT, STAGO). The TG was expressed as endogenous thrombin potential (ETP) in nmol×min.
2.3.4Hemostatic balanceHemostatic balance was assessed by means of procoagulant to anticoagulant factor ratios and ETP ratio with/without PC activator.
2.4Statistical analysisStatistical Package for Social Science (SPSS) software version 21.0 (IBM Corp., Armonk. New York, USA) was used for analysis of data. Data were summarized as mean and percentage. Study groups were compared using the χ2 test for qualitative variables. Student's t and ANOVA tests were used for analysis of parametric data. Mann–Whitney U and Kruskal–Wallis H tests were used for analysis of non-parametric data. p-Value less than or equal to 0.05 was considered significant.
3ResultsMean age of patients at inclusion was 57.8 years (16–91 years). Mean age at diagnosis of cirrhosis was 55 years (6–88 years). There were 24 males and 27 females. Major etiology of cirrhosis was viral hepatitis (64.7%). Patients were categorized according to Child–Pugh score as class A in 14 patients (27.5%), class B in 22 patients (43.2%), and class C in 15 patients (29.3%). (H+) group included 9 patients (17.6%). Bleeding episodes were variceal hemorrhage (n=6), gastrointestinal bleeding secondary to gastric (n=1) and duodenal (n=1) ulcers and gingivorrhagia (n=1). Hemorrhage was judged mild to moderate in 6 patients and severe in 3 others. Both patient groups were comparable regarding age, sex and Child–Pugh score.
Obviously, as regards controls, no bleeding episodes occurred within 2 months before or after inclusion.
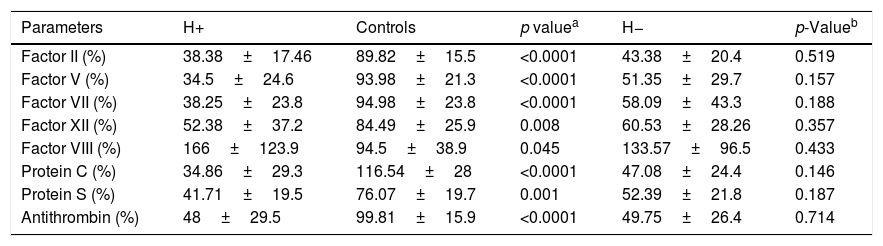
3.1Conventional laboratory testsThere was no significant difference in mean levels of PT, aPTT and fibrinogen between (H+) and (H−) groups. Platelet count was significantly lower in (H+) patients than in (H−) patients (p=0.01) (Table 1).
Comparison between patients groups as regards conventional laboratory tests.
| Parameters | H+ | H− | p-Value |
|---|---|---|---|
| Platelet count (/mm3) | 62,888±33,650 | 110,317±70,483 | 0.01a |
| PT (%) | 52.7±10.8 | 62±21.3 | 0.1 |
| aPTT (ratio) | 1.40±0.3 | 1.26±0.3 | 0.16 |
| Fibrinogen (g/L) | 2.12±0.6 | 2.6±0.9 | 0.21 |
H+: cirrhotic patients who experienced bleeding; H−: cirrhotic patients who did not experienced bleeding; PT: prothrombin time; aPTT: activated partial thromboplastin.
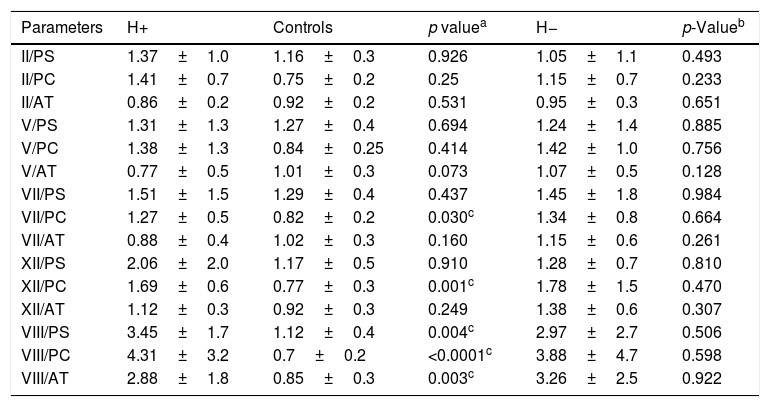
Mean levels of pro-and anticoagulant factors were significantly lower in cirrhotic patients who experienced bleeding episodes (H+) than in controls. However, factor VIII was significantly higher in cirrhotic patients than in controls (166% vs. 94.5%; p=0.045). There was no significant difference between both (H+) and (H−) groups as regards levels of pro- and anticoagulant factors (Table 2).
Comparison between patients groups and controls as regards procoagulant and anticoagulant factors.
| Parameters | H+ | Controls | p valuea | H− | p-Valueb |
|---|---|---|---|---|---|
| Factor II (%) | 38.38±17.46 | 89.82±15.5 | <0.0001 | 43.38±20.4 | 0.519 |
| Factor V (%) | 34.5±24.6 | 93.98±21.3 | <0.0001 | 51.35±29.7 | 0.157 |
| Factor VII (%) | 38.25±23.8 | 94.98±23.8 | <0.0001 | 58.09±43.3 | 0.188 |
| Factor XII (%) | 52.38±37.2 | 84.49±25.9 | 0.008 | 60.53±28.26 | 0.357 |
| Factor VIII (%) | 166±123.9 | 94.5±38.9 | 0.045 | 133.57±96.5 | 0.433 |
| Protein C (%) | 34.86±29.3 | 116.54±28 | <0.0001 | 47.08±24.4 | 0.146 |
| Protein S (%) | 41.71±19.5 | 76.07±19.7 | 0.001 | 52.39±21.8 | 0.187 |
| Antithrombin (%) | 48±29.5 | 99.81±15.9 | <0.0001 | 49.75±26.4 | 0.714 |
H+: cirrhotic patients who experienced bleeding; H−: cirrhotic patients who did not experienced bleeding.
There was no significant difference between (H+) patients and controls as regards ETP mean level without PC activator (716.1 vs. 811nmol×min respectively; p=0.5). In the presence of PC activator, ETP was surprisingly higher in (H+) patients than in controls (725 vs. 387nmol×min; p=0.05).
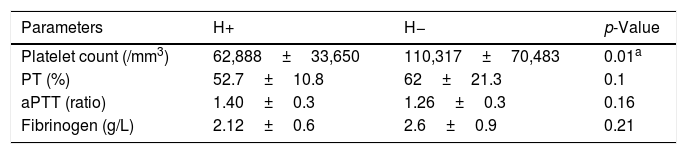
However, there was no significant difference between patient groups as regards ETP with and without PC activator.
3.4Hemostatic balance3.4.1The ratios of pro- to anticoagulant factorsCompared with controls, VIII/anticoagulant factors (VIII/PC, VIII/PS, VIII/AT), VII/PC and XII/PC were significantly higher in (H+) patients. All other ratios were roughly at the same levels.
No significant difference as regards all ratios between patients who experienced bleeding (H+) and those who did not (H−) was detected (Table 3).
Comparison between patients groups and controls as regards ratios of pro to anticoagulant factors.
| Parameters | H+ | Controls | p valuea | H− | p-Valueb |
|---|---|---|---|---|---|
| II/PS | 1.37±1.0 | 1.16±0.3 | 0.926 | 1.05±1.1 | 0.493 |
| II/PC | 1.41±0.7 | 0.75±0.2 | 0.25 | 1.15±0.7 | 0.233 |
| II/AT | 0.86±0.2 | 0.92±0.2 | 0.531 | 0.95±0.3 | 0.651 |
| V/PS | 1.31±1.3 | 1.27±0.4 | 0.694 | 1.24±1.4 | 0.885 |
| V/PC | 1.38±1.3 | 0.84±0.25 | 0.414 | 1.42±1.0 | 0.756 |
| V/AT | 0.77±0.5 | 1.01±0.3 | 0.073 | 1.07±0.5 | 0.128 |
| VII/PS | 1.51±1.5 | 1.29±0.4 | 0.437 | 1.45±1.8 | 0.984 |
| VII/PC | 1.27±0.5 | 0.82±0.2 | 0.030c | 1.34±0.8 | 0.664 |
| VII/AT | 0.88±0.4 | 1.02±0.3 | 0.160 | 1.15±0.6 | 0.261 |
| XII/PS | 2.06±2.0 | 1.17±0.5 | 0.910 | 1.28±0.7 | 0.810 |
| XII/PC | 1.69±0.6 | 0.77±0.3 | 0.001c | 1.78±1.5 | 0.470 |
| XII/AT | 1.12±0.3 | 0.92±0.3 | 0.249 | 1.38±0.6 | 0.307 |
| VIII/PS | 3.45±1.7 | 1.12±0.4 | 0.004c | 2.97±2.7 | 0.506 |
| VIII/PC | 4.31±3.2 | 0.7±0.2 | <0.0001c | 3.88±4.7 | 0.598 |
| VIII/AT | 2.88±1.8 | 0.85±0.3 | 0.003c | 3.26±2.5 | 0.922 |
PS: protein S; PC: protein C; AT: antithrombin.
ETP ratio with/without PC activator was significantly higher in (H+) patients than in controls (0.97 vs. 0.51; p=0.017) (Fig. 1A).
However, there was no significant difference between patient groups as regards ETP ratio (Fig. 1B).
4DiscussionThe current study showed that the hemostatic balance assessed by ratios of pro- to anticoagulant factors and ETP ratio with/without PC activator was in favor of a hypercoagulability state even in cirrhotic patients who experienced bleeding events. When comparing both patient groups, cirrhotic patients who had bleeding generated roughly as much thrombin as those who had not. To our knowledge, there were no published studies investigating the impact of thrombin generation assay in assessing the bleeding risk in cirrhosis.
However, the main limitation of this study is the small number of cirrhotic patients who experienced bleeding.
The bleeding tendency in cirrhotic patients namely in those with advanced liver disease was supported by many clinical observations [5]. The most relevant hemorrhagic events in such patients result from esophageal varices rupture and portal hypertensive gastropathy [6]. Bleeding may occur also from the same lesions seen in the general population such as peptic ulcer, epistaxis, gingivorrhagia, bruising or from invasive procedures. In our study, variceal bleeding was the most common site of hemorrhage as it occurred in two-thirds of patients.
Historically, hemostatic abnormalities due to liver failure such as prolonged PT and aPTT were assumed to reflect bleeding disorders that frequently occur in cirrhotic patients. Thus, PT has frequently been corrected by means of fresh frozen plasma prior to liver biopsy or other potentially hemorrhagic procedures [7].
In the present study, there was not any significant difference between patients who experienced bleeding and those who did not as regards PT and aPTT mean levels. Our results are in accordance with those of many papers suggesting these conventional tests are not as good at predicting bleeding in acquired as compared with congenital coagulopathies [8]. In fact, Segal et al. found no evidence to conclude that prolonged PT could predict bleeding during invasive diagnostic procedures [9]. Furthermore, Tripodi noted that, paradoxically, cirrhotic patients with near-normal PT could bleed whereas patients with relatively abnormal PT commonly did not [10]. The lack of its bleeding predictive power can best be explained by the fact that only the procoagulant but not the anticoagulant activity (PC, PS, AT) is being reflected by PT [11]. Since both procoagulant and anticoagulant factors are decreased in cirrhosis, PT and aPTT could not represent the balance between pro-and anticoagulant factors [5].
With regard to platelet count assessed in the present study, patients who experienced bleeding had significantly lower levels than patients who did not (p=0.01). Our results were in agreement with those of El Bokl et al. where platelet count was found to be significantly lower in cirrhotic patients who experienced hematemesis and melena than those who did not have bleeding episodes (p<0.0001) [3]. These findings could be supported by the multiple quantitative and qualitative alterations that could affect platelets in patients with chronic liver disease. Increased platelet sequestration in the spleen caused by portal hypertension and decreased thrombopoietin production by the diseased liver are main mechanisms of thrombocytopenia in cirrhotic patients [12]. Besides thrombocytopenia, defective platelet function has also been found since adhesion, aggregation and then capacity to support thrombin generation are impaired in cirrhotic patients. These findings were supported by several clinical observations showing that a platelet count <60,000/mm3 was associated with a significantly increased risk of bleeding related to invasive procedures [13]. Thus, platelet count seems to be more reliable than other hemostatic conventional tests (TP or aPTT) in assessing bleeding risk. Although there is no prospective studies determining the threshold platelet count for prophylactic transfusion in cirrhotic patients, AASLD guidelines for liver biopsy recommend consideration of platelet transfusion prior to liver biopsy for a platelet count <50,000–60,000/mm3 (Class I level C) [7].
In the current study, there was not any difference between (H+) and (H−) patients with regard to plasma fibrinogen level. However, Siddiqui et al. found significant correlation for decreased fibrinogen level with gastrointestinal bleeding in cirrhotic patients (RR=1.47; 95% CI, 0.64–3.35) [4].
In fact, hypofibrinogenemia which is frequently seen in patients with advanced cirrhosis is thought to increase bleeding risk since fibrinogen serves both as a precursor of fibrin and a mediator of platelet aggregation [14]. Bleeding risk is considered particularly high when fibrinogen levels are below 1g/L [15]. In the present study, our results as regards fibrinogen levels could be explained by the fact that the majority of patients had stable liver disease.
In the present study, procoagulant (II, V, VII and XII) and anti-coagulant factors (PC, PS and AT) were significantly lower in cirrhotic patients who had bleeding than in controls. However, FVIII was markedly increased in the former group. Several previous studies were in agreement with our results regardless of bleeding or thrombotic status of cirrhotic patients [16–18].
When comparing (H+) and (H−) patients, no difference in levels of pro-and anticoagulant factors was detected. In line with our results, El Bokl et al. showed no significant difference between both patient groups as regards FVIII level. However, paradoxically, PC was significantly lower in cirrhotic patients who experienced bleeding in comparison with those who did not (36.4% vs. 65%; p=0.018) [3]. These findings suggest that bleeding events in cirrhotic patients are not related to plasma levels of coagulation factors. Indeed, randomized controlled trials provide evidence against effectiveness of recombinant activated factor VII infusion in controlling bleeding from varices or during hepatectomy [19,20].
In the present study, regarding hemostatic balance, VIII/anticoagulant factors (VIII/PC; VIII/PS and VIII/AT), VII/PC and XII/PC were significantly higher in (H+) patients than in controls. All other ratios were roughly at the same levels between (H+) patients and controls. Similarly, previous studies showed that ratios pro- to anticoagulant factors (II/PC, V/PC, VIII/PC and VIII/AT) were significantly higher in cirrhotic patients than in controls [16,17,21].
In the current study, all ratios of pro- to anticoagulant factors were at the same levels between both patient groups. However, El Bokl et al. showed paradoxically a significant higher level of FVIII/PC in cirrhotic patients who had bleeding events than in patients who had not [3].
With regard to thrombin generation assessed in the current study, there was no difference across controls and patient groups.
After activation of PC, ETP ratio was significantly higher in (H+) patients than in controls (p=0.017). These findings were in agreement with those of Tripodi et al. suggesting a state of resistance to the anticoagulant effect of activated PC in cirrhotic patients [17,18]. This “hypercoagulability status” could even have a protective effect against bleeding in cirrhotic patients. In the same context, ETP ratio did not reach statistical significance between both patient groups. This finding provides an additional argument against the traditional tenet holding that coagulation disorders detected in cirrhotic patients are predictors of bleeding.
It appears that gastrointestinal bleeding may be explained by hemodynamic alterations related to portal hypertension. Advanced liver disease, large varices, variceal wall tension and the presence of red wale marking have been reported as risk factors for variceal bleeding in cirrhotic patients [22]. Endothelial dysfunctions, bacterial infections and renal failure may also contribute to gastrointestinal bleeding [23]. Other bleeding events such as epistaxis, gingivorrhagia and bruising may be explained by thrombocytopenia, which is in turn mainly due to portal hypertension [23].
Before concluding, it is worth noting that in patients with chronic liver disease, vitamin k deficiency is one of the main factors implicated in coagulation disorders described above. Indeed, vitamin K deficiency may result in, not only decreased synthesis of coagulation factors but also in producing under-carboxylated precursors of these proteins that are functionally inactive. These proteins are known as proteins induced by vitamin K absence (PIVKA) such as undercaboxylated prothrombine (PIVK-II) which has been shown to be secreted by hepatocellular carcinoma cells in cirrhotic patients [24,25]. Although it has been reported in some previous studies that the administration of vitamin K in such patients did not improve coagulation disorders, data regarding the sensitivity of under-carboxylated prothrombin are still conflicting [26,27]. Hence, we could speculate that it would probably be wise not to administer vitamin K in cirrhotic patients in order to prevent a significant decrease of the level of undercarboxylated prothrombin which may play a role in the early diagnosis of hepatocellular carcinoma in such patients [28].
In conclusion, although our findings should be interpreted cautiously due to the small number of (H+) patients, they may support the concept that abnormal conventional laboratory tests such as prolonged PT and aPTT do not correlate with bleeding risk in cirrhotic patients. However, platelet count seems to be a better predictor of bleeding than PT and aPTT. The hemostatic balance is shifted toward a hypercoagulability state even in cirrhotic patients who experienced bleeding. We surmise that gastrointestinal bleeding in such patients is mainly hemodynamic due to portal hypertension. Further studies should take into account the fibrinolytic system. In fact, hyperfibrinolysis has been reported in some cases as a cause of bleeding in cirrhotic patients, although this concept remains controversial [10].AbbreviationsETP
endogenous thrombin potential
PCprotein C
PTprothrombin time
aPTTactivated partial thromboplastin
PPPpoor platelet plasma
PSprotein S
ATantithrombin
TGthrombin generation
Copyright assignmentIn consideration of the Fundación Clínica Médica Sur (FCMS) taking action to review and edit my submission, the undersigned authors, jointly and severally, hereby transfer, convey, and assign all right, title, and interest therein, including any and all copyrights in all forms and media now or hereafter known, to the FCMS. The authors retain the nonexclusive right to use all or part of the Article in future works of their own in a noncompeting way, provided proper copyright credit is given to the Foundation. Should the FCMS not publish the aforesaid submission, the FCMS agrees to release its rights therein (Note: material prepared by employees of the federal government in their official duties may not be copyrightable). No guarantee is made that the Article will be published.
Authorship responsibilityI, the undersigned author, certify that I have participated sufficiently in the intellectual content, the analysis of data, if applicable, and the writing of the Article, to take public responsibility for it. I have reviewed the final version of the Article, believe it represents valid work, and approve it for publication. As an author of this Article, I certify that none of the material in the manuscript has been published previously, is included in another manuscript, or is currently under consideration for publication elsewhere. I also certify that the Article has not been accepted for publication elsewhere, nor have I assigned any right or interest in the Article to any third party.
Financial disclosureI, the undersigned author, certify that I have no commercial associations (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements) that might pose a conflict of interest in connection with the submitted article. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board/Animal Care Committee ApprovalI, the undersigned author, certify that my institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Author contributionsAsma labidi, Hela Baccouche, Monia Fekih and Neila BenRomdhane designed the study.
Hela Baccouche carried out biological tests.
Asma Labidi and Hela Baccouche analyzed the data.
Asma labidi wrote the manuscript with the support of Hela Baccouche.
All authors discussed the results and contributed to the final manuscript.
Conflict of interestNone.
Informed consentAll patients gave their informed consent for publication of the case details.