The gut microbiota has been considered a cornerstone of maintaining the health status of its human host because it not only facilitates harvesting of nutrients and energy from ingested food, but also produces numerous metabolites that can regulate host metabolism. One such class of metabolites, the bile acids, are synthesized from cholesterol in the liver and further metabolized by the gut microbiota into secondary bile acids. These bioconversions modulate the signaling properties of bile acids through the nuclear farnesoid X receptor and the G protein-coupled membrane receptor 5, which regulate diverse metabolic pathways in the host. In addition, bile acids can regulate gut microbial composition both directly and indirectly by activation of innate immune response genes in the small intestine. Therefore, host metabolism can be affected by both microbial modifications of bile acids, which leads to altered signaling via bile acid receptors, and by alterations in the composition of the microbiota. In this review, we mainly describe the interactions between bile acids and intestinal microbiota and their roles in regulating host metabolism, but we also examine the impact of bile acid composition in the gut on the intestinal microbiome and on host physiology.
The human intestinal tract harbors a diverse and complex microbial community that plays a key role in human health. It has been estimated that our gut contains more than 1,000 phylotypes that contain 100-fold more genes than are found in the human genome.1 This gut microbi-ota (GM) is composed of bacteria, archaea, viruses, and fungi, which are divided into six divisions/phyla: Firmi-cutes, Bacteroidetes, Proteobacteria, Acinetobacteria, Fusobacteria, and Verrucomicrobia.2,3 The vast majority of bacteria comprising the GM are obligate anaerobes with lesser numbers of facultative anaerobes, archaea, and yeast species. Firmicutes and Bacteroidetes make up more than 90% of the overall GM. The most frequent genera of obligate anaerobes include Bacteroides, Bifidobacterium, Clostridium, Eubac-terium, Fusobacterium, Peptococcus, Peptostreptococcus, and Rumminococcus. The genera of facultative anaerobic bacteria include Escherichia, Enterobacter, Enterococcus, Klebsiella, Lactobacillus, and Proteus.4,5
The Marriage: In Sickness and in HealthIt is well established that a healthy GM is important for the overall health of the host, because the composition of the GM has an immense impact on human well-being, including host metabolism, physiology, nutrition, and immune function.6 The composition and function of the GM differs according to geographical location, age, sex, and the mother’s microbiota. The simple community of microbes present at birth gradually develops into a diverse ecosystem during host growth. Over time, many host-bacterial associations have developed into beneficial relationships.7 Symbiotic bacteria metabolize indigestible compounds, supply essential nutrients, defend against colonization by opportunistic pathogens, and contribute to the formation of the intestinal architecture.8 Some studies have shown that a number of factors play a role in shaping the normal GM, including the mode of delivery at birth (cesarean or vaginal), diet during infancy (breast milk or formula) and in adulthood (meat based or vegan/vegetarian), plus the use or presence of antibiotic-like molecules derived from the environment or the gut commensal community.
In contrast, an imbalance in the equilibrium of the GM (dysbiosis) can predispose to a range of different types of diseases at different ages, ranging from allergies in childhood to inflammatory bowel disease (IBD) in young adults.9 Dysbiosis can result from exposure to diverse environmental factors, including diet, drugs, toxins, and pathogens, and is negatively associated with the host’s health, leading to host susceptibility to diseases such as diabetes, IBD, and metabolic syndrome.10
Gut MicrobiotaIt has long been known that humans are colonized by a range of microorganisms, most of which are present in the intestinal tract where they form a complex microbial community known as the intestinal or gut microbiota (GM).11 The establishment of the GM occurs during childhood, and the nature of the microbiota in the human intestine during the early stages of life plays a key role in the maturation and modulation of the host immune system and in the promotion of various physiological processes in the human intestine, including the regulation of intestinal barrier integrity and the secretion of mucus.12-14 In adult life, the GM performs a variety of functions for the maintenance of human health, for example, assisting in food degradation, releasing nutrients, promoting the differentiation of certain host tissues, reducing the risk of intestinal colonization by pathogens, and modulating the immune system.15
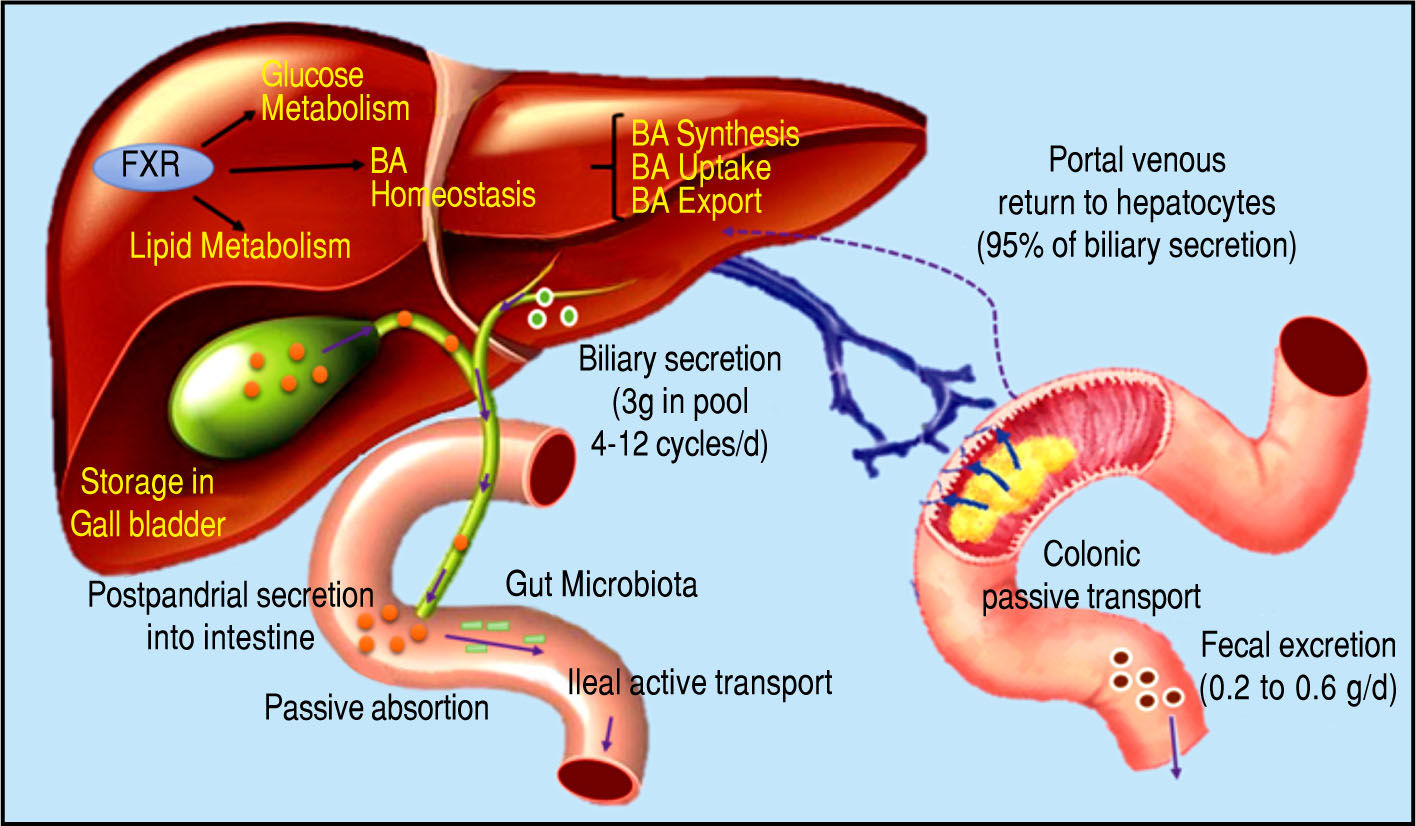
Bile Acids and Gut MicrobiotaBile acids (BA) are amphipathic molecules synthesized in the liver from cholesterol, which are stored in the gallbladder and released into the small intestine after food in-take16 (Figure 1). They have many fundamental roles but one of their major functions is to facilitate the emulsifica-tion of dietary fats and to assist the intestinal absorption of lipids and lipophilic vitamins.17 Recently, it has been recognized that BA are signaling molecules for a variety of activities mediated through the farnesoid X receptor(FXR) and the G protein-coupled membrane receptor 5 (TGR5). These receptors mediate the signaling cascade and activate expression of genes involved in the metabolism of BA, li-pids, and carbohydrates and in energy expenditure and inflammation, predominantly in enterohepatic tissues but also in peripheral organs.18,19
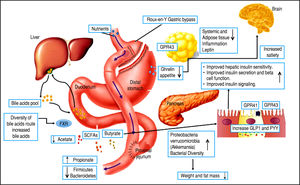
Bile acid synthesis and enterohe-patic circulation. The human bile acid (BA) pool consists of approximately 3 g of BA. Food intake stimulates the gallbladder to release BA into the small intestine. Humans produce on average about 0.5 g BA per day by synthesis in the liver, and secrete approximately 0.5 g/day. Conjugated BA are efficiently reabsorbed from the ileum by active transport, and a small amount of unconjugat-ed BA is reabsorbed by passive diffusion in the small and large intestines. The first-pass extraction of BA from the portal blood by the liver is very efficient.
The human BA pool consists of the primary colic and chenodeoxycholic acids and the secondary deoxycholic and lithocolic acids.20 It is known that synthesis of BA is a multi-step process that involves diverse enzymes located in the endoplasmic reticulum, mitochondria, cytosol, and peroxisomes of cells. BA synthesis from cholesterol can occur via two pathways: the classical pathway, which occurs in hepatocytes and is known as the neutral route, and the alternative pathway, which occurs in the gut.21 The classical pathway mediates synthesis of primary BA and represents more than 90% of BA synthesis, which is why it is considered the main route of BA synthesis.22 The alternative way is responsible for synthesis of secondary BA and mediates less than 10% of BA synthesis under normal physiological conditions.23
In this context, it is important to review the enterohe-patic circulation of BA to understand the key role of these molecules. Conjugated BA are secreted across the canalic-ular membrane into the bile and stored in the gall bladder. After a meal, the duodenum secretes cholecystokinin, which stimulates the contraction of the gallbladder and thereby releases BA into the intestinal tract. Within the small intestine, micellar BA act as effective detergents to facilitate the solubilization of monoacylglycerols and fatty acids, and the digestion and absorption of dietary lipids and fat-soluble vitamins. Finally, BA are reabsorbed in the ileum and conveyed back to the liver through the portal blood for re-secretion into the bile.24-28
Hepatic BA transport requires active transport systems because BA cannot cross the hepatocyte membrane. Most circulating BA are taken up by hepatocytes via Na+-de-pendent cotransport systems. The Na+-dependent tauro-cholate transporter has been identified as the major BA uptake transporter in the basolateral membrane of hepato-cytes.29 The results of studies in mice by Fretland, et al.30 suggested a role for the microsomal epoxide hydrolase (mEH) in regulating basolateral Na-dependent BA uptake, however, a study by Zhu, et al. demonstrated that a point mutation that resulted in significantly decreased mEH expression in a human individual led to hyper-cholanemia, a condition in which BA levels are increased in plasma in the absence of hepatocyte injury, suggesting impaired basolateral BA uptake rather than intrahepatic BA accumulation.31 It has been estimated that more than 25% of BA uptake by hepatocytes is regulated through Na+-in-dependent transporters (Organic anion transporters: OATP1A2, OATP1B1 and OATP1B3).32 This pathway is primarily responsible for the uptake of unconjugated BA.
However, FXR, a nuclear receptor activated by BA, plays an important role in BA homeostasis by controlling the expression of genes for proteins including the nuclear receptor small heterodimer partner.33
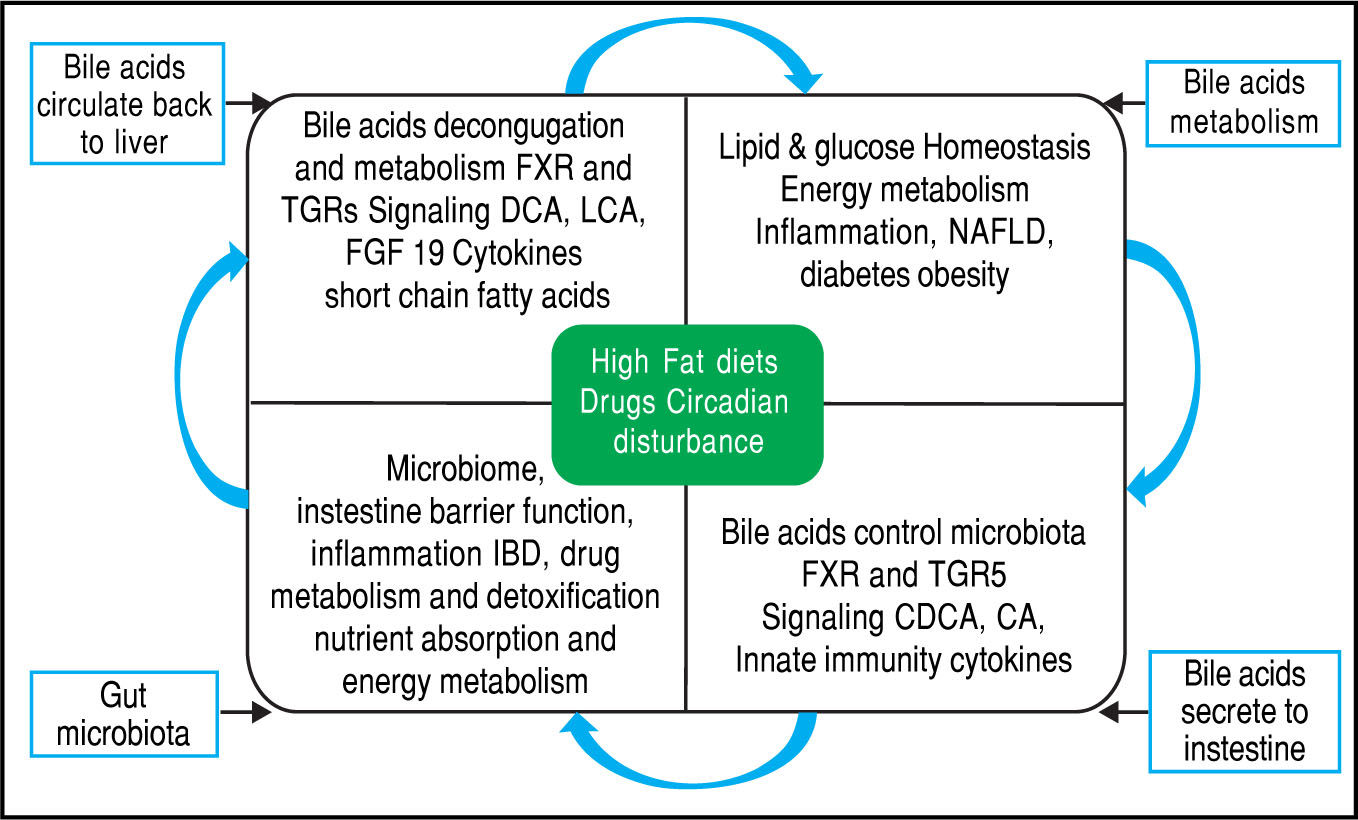
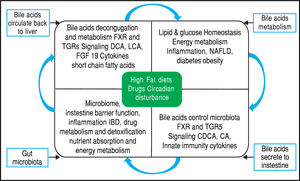
Equally importantly, it has been observed that the GM is involved in the biotransformation of BA through decon-jugation, dehydroxylation, and reconjugation of these mol-ecules.34 Moreover, it has been recognized that BA has antimicrobial activity that can damage bacterial cell membranes and thus inhibit bacterial overgrowth.35 BA can also regulate the overgrowth and composition of the intestinal microbiota through FXR and TGR-5 to protect the liver and intestine against inflammation36 (Figure 2). A study in humans by David, et al. showed that an animal-based diet rapidly altered the GM, increasing the number of bile-tolerant microorganisms (B. wadsworthia and Bacteroides) and decreasing the number of Firmicutes.37 The results of this study suggested a relationship between dietary fat, BA, and the overgrowth of microorganisms in IBDs such as Crohn’s disease.38
Bidirectional interactions between bile acid synthesis and gut microbiota. The relationship between bile acids and the gut microbio-ta is close and complementary. Bile acids control gut bacteria overgrowth and protect against inflammation while the gut microbiota plays a role in biotransformation of bile acids and affects bile acid composition and metabolism via Farnesoid X Receptor and G protein-coupled membrane receptor 5 signaling in the liver.
A healthy GM is crucial for normal metabolic function and host homeostasis. Alterations in the composition of the GM may be related to obesity by modifying the reservoir metabolism and the mechanism of appetite.39 Some studies have shown a close relationship between obesity and the composition of the GM. In 2005, Ley, et al. demonstrated that metabolic dysfunction was related to changes in the Bacteroidetes/Firmicutesratio. They analyzed 5,088 bacterial 16S rRNA gene sequences from the distal intestinal microbiota of genetically obese ob/ob mice, lean ob/+ and wild-type siblings, and their ob/+ mothers, all fed with a polysaccharide-rich diet. They observed that compared the lean mice, regardless of lineage, the ob/ob animals had a 50% reduction in the amount of Bacteroidetes and a proportional increase in Firmicutes. Based on40 In contrast, a study in humans by Walter, et al. concluded that the strong relationship between microbiome changes and obesity that is observed in mice does not apply in humans, because no significant differences in the Bacteroidetes/Firmicutes ratio were observed between obese and non-obese individuals.38
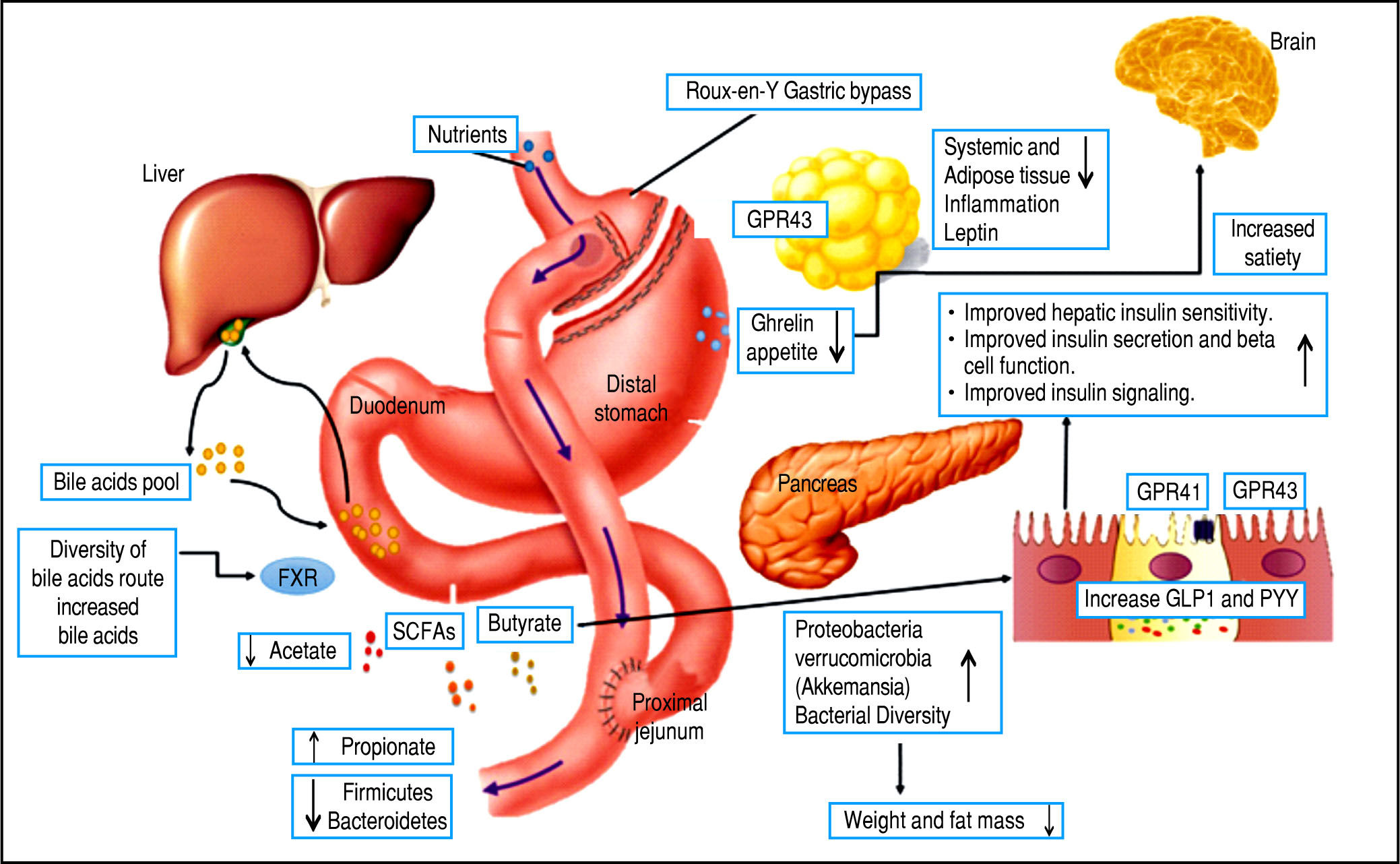
However, there are several well-described interactions between the GM and the host. One of these is the gut-brain axis, which indirectly influences the nature of the commensal organisms, gastrointestinal motility and secretion, and intestinal permeability, and directly, via signaling molecules released into the gut lumen from cells in the lamina propria, modifying levels of plasma peptides: mainly glucagon-like peptide 1 (GLP-1) and peptide YY (PYY).41-43 Analysis of the importance of these hormones has focused on obese patients undergoing bariatric surgery, who show increased levels of GLP-1 and PYY post-prandially. Significantly, inhibiting the PYY and GLP-1 responses resulted in the return of appetite and increased food intake. Therefore, it is likely that elevated levels of PYY and GLP-1 play a key role in the sustained weight loss observed following gastric bypass surgery.44-47
Bariatric surgery, a range of minimally invasive laparos-copy procedures used to treat severe cases of obesity including gastric banding, Roux-en-Y gastric bypass (RYGB), gastric sleeve, and biliopancreatic diversion,48-49 has shown success in changing the GM, resulting in a greater abundance of Gammaproteobacteria and Verrucomicrobia (Akkermansia) together with a reduced abundance of Fir-micutes50,51 and importantly, increased GLP-1 and PYY levels. These are promising results for the use of bariatric surgery to treat obese patients and their metabolic complications; however, more studies are necessary to assess the safety of these procedures52,54 (Figure 3).
ConclusionThe GM can be modified by age, diet, drugs, and disease. BAs appear to be a principal regulator of the GM. In addition, the size of the BA pool has been shown to be a function of microbial metabolism in the intestines, although the majority of these studies have been performed in mice. Consequently, there is a lack of evidence for this link in humans, which makes it clear that further studies are necessary to identify new therapeutic targets for maintaining human intestinal health.
Abbreviations- •
BA: bile acids.
- •
FXR: Farnesoid X Receptor.
- •
GLP-1: glucagon-like peptide 1.
- •
GM: gut microbiota.
- •
IBD: inflammatory bowel disease.
- •
mEH: microsomal epoxide hydroxide.
- •
OATP: Organic anion transporter.
- •
PYY: peptide YY.
- •
RYGB: Roux-en-Y gastric bypass.
- •
TGR5: G protein-coupled membrane receptor 5.