Research has shown that hepatitis B (HBV) and Delta virus (HDV) are a worldwide public health problem. This study aims to estimate the prevalence rates of HBV and HDV infection in five municipalities of Maranhão, Northeastern Brazil.
Materials and methodsA total sample between 3856 and 4000 individuals. Questionnaires were used to register sociodemographic characteristics and factors associated with transmission. Patients were tested for hepatitis B virus surface antigen (HBsAg), anti-hepatitis B core antigen (anti-HBc), and antibodies against hepatitis Delta virus (anti-HDV). Factors associated with HBV were detected by means of multivariate Poisson regression.
ResultsOverall, 3983 subjects were included. Ninety-two of the participants were HBsAg-positive (2.30%, 95% CI 1.80–2.80), and anti-HBc was detected in 1535 (38.50%, 95% CI 37–40). The factors associated with the presence of anti-HBc were: (1) Municipality (P<0.001); Age (P<0.001); School education (P<0.001); Illicit drug use (P=0.001); non-HBV vaccine (P=0.041). Among the HBsAg carriers, eight were anti-HDV-positive (8.69%, 95% CI 2.90–14.40). The most frequent HBV genotype was D4. The only HDV genotype was HDV-8.
ConclusionHBV exhibited intermediate endemicity in the studied region. Traditional factors were associated with exposure to the virus. The presence of the HDV was confirmed. The most frequent HBV and HDV genotypes were unlike the ones currently described in Brazil.
In 2015, 257 million people were living with chronic HBV [1]. In Brazil, a recent national survey (2004–2009) in all state capitals found HBsAg seroprevalence rates of 0.63%, 0.48%, 0.37%, 0.31%, 0.31% and 0.26% in the North, South, Northeast, Central-West and Southeast regions and in the Federal District, respectively [2,3]. The results of the above study were included in the review of Ott et al. (2012) and Schweitzer et al. (2015) and were pivotal for defining Brazil as a country with low endemicity [4].
One complicating factor in chronic HBV is co-infection with HDV in which the prevalence of HDV among HBV carriers correspondes to 48–60 million infections globally. [5,6].
HDV main transmission route is parenteral and requires HBsAg for infectivity [7,8]. Its global distribution is heterogeneous; however, despite its link to HBV, their endemicity rates do not always coincide [9,10].
Until recently in Brazil, HDV was thought to be present only in the Western Amazon. However, after studying a sample of HBV-positive individuals in Maranhão State, five cases of HBV-HDV co-infection were detected among patients from a specific region of the state, and surprisingly, the HDV-8 genotype was identified, which had never been described among individuals born outside Africa [11]. In the above population, the HBV genotype D4 was also found in co-infections with HDV-8, suggesting that slave trade might have been responsible for the introduction of these viruses in Maranhão State [12]. Thus, the present study conducted a population-based survey in five municipalities of the above-mentioned region, aiming to estimate the prevalence rates of HBV and HDV infection and to determine the associated factors.
2Materials and methods2.1Study populationThe present work is a population-based prevalence study conducted in the Northeast region of Brazil, in the municipalities Urbano Santos (24,573 inhabitants), Axixá (11,407 inhabitants), Morros (17,783 inhabitants), Icatu (25,145 inhabitants) and Humberto de Campos (26,189 inhabitants) of Maranhão State, from March 2012 to June 2016.
Subjects with at least one year of age and living for at least six months in the studied municipalities were included. The sample was calculated using a 0.5% prevalence of HBsAg (considering the result of the national survey on HBsAg prevalence in the Northeast region [6]), a 0.3% absolute error, a 95% confidence interval (95% CI) and a design effect of 2, thus totaling an estimated sample from 3856 to 4000 individuals.
Participants were selected via cluster sampling, for which municipalities were divided into sectors. With a map of each sector, the first block was drawn, followed by the starting point of the block and then the route. If a block did not render enough samples for the sector, an additional block was drawn, and this cycle was repeated until the sample size of each sector was achieved.
2.2Data collectionIndividual data on socioeconomic and demographic variables, risk factors, alcohol use and hepatitis B vaccination status (assessed by viewing the participant's vaccination card) were collected by trained interviewers who administered a structured questionnaire during home visits.
2.3Laboratory testsBlood samples collected after the interview were submitted to enzyme-linked immunosorbent assay (ELISA) with commercial kits (Diasorin®, Italy) to detect HBsAg, anti-hepatitis B core antigen (anti-HBc), anti-HBs and anti-HDV in HBsAg-positive samples.
2.4Statistical analysisIn the data analysis, anti-HBc was the dependent variable. The relative risk was estimated by means of a multivariate Poisson regression with a robust variance fit, and 95% CIs were calculated.
Variables were selected for the model if they exhibited P<0.2 in the non-fitted analysis. There were no criteria for the removal of variables. Since more than one person per home could be interviewed, estimates were corrected for clustering or agglomeration. Data analysis was performed with Stata® software, version 12.0 (Stata Corp., College Station, TX, USA). The level of significance was set at 0.05.
2.5Ethics StatementThis project was approved by the Human Research Ethics Committee of the University Hospital of the Federal University of Maranhão under number 448.731. All adult subjects provided written consent, and a parent or guardian of any child participant provided informed consent on behalf of the child.
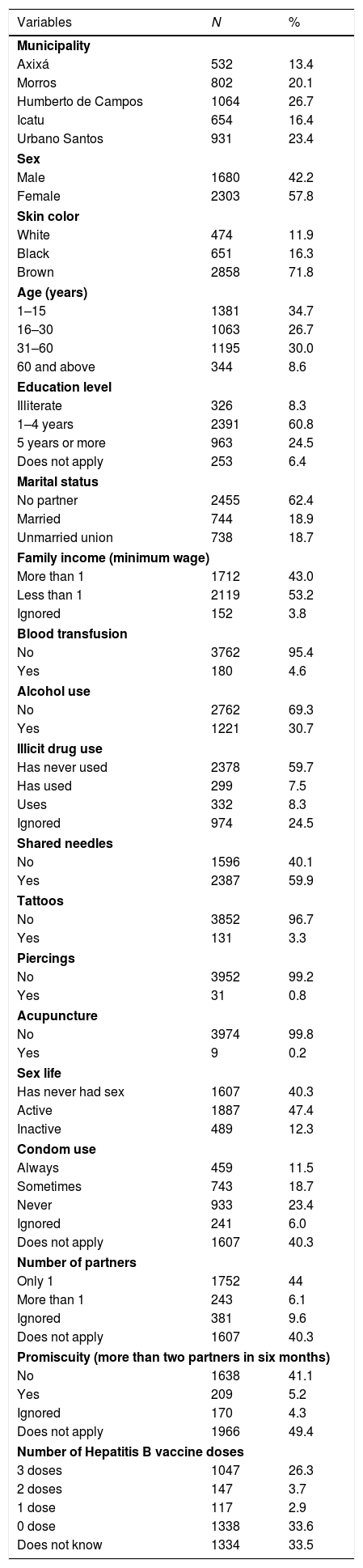
3ResultOverall, 3983 subjects were included in the study: 1496 (37.6%) were living in urban and 2487 (62.4%) in rural areas. Table 1 shows the frequency distributions of demographic (municipalities), socioeconomic (sex, skin color, age, education, marital status and family income) and epidemiological factors (history of blood transfusion, alcohol use, drug use, needle sharing, tattoos, piercings, acupuncture, sex life, condom use, number of partners and number of hepatitis B vaccine doses) in the studied population.
Profile of demographic, socioeconomic and epidemiological variables of hepatitis B. Maranhão State, Brazil, 2012–2016 (n=3983).
| Variables | N | % |
|---|---|---|
| Municipality | ||
| Axixá | 532 | 13.4 |
| Morros | 802 | 20.1 |
| Humberto de Campos | 1064 | 26.7 |
| Icatu | 654 | 16.4 |
| Urbano Santos | 931 | 23.4 |
| Sex | ||
| Male | 1680 | 42.2 |
| Female | 2303 | 57.8 |
| Skin color | ||
| White | 474 | 11.9 |
| Black | 651 | 16.3 |
| Brown | 2858 | 71.8 |
| Age (years) | ||
| 1–15 | 1381 | 34.7 |
| 16–30 | 1063 | 26.7 |
| 31–60 | 1195 | 30.0 |
| 60 and above | 344 | 8.6 |
| Education level | ||
| Illiterate | 326 | 8.3 |
| 1–4 years | 2391 | 60.8 |
| 5 years or more | 963 | 24.5 |
| Does not apply | 253 | 6.4 |
| Marital status | ||
| No partner | 2455 | 62.4 |
| Married | 744 | 18.9 |
| Unmarried union | 738 | 18.7 |
| Family income (minimum wage) | ||
| More than 1 | 1712 | 43.0 |
| Less than 1 | 2119 | 53.2 |
| Ignored | 152 | 3.8 |
| Blood transfusion | ||
| No | 3762 | 95.4 |
| Yes | 180 | 4.6 |
| Alcohol use | ||
| No | 2762 | 69.3 |
| Yes | 1221 | 30.7 |
| Illicit drug use | ||
| Has never used | 2378 | 59.7 |
| Has used | 299 | 7.5 |
| Uses | 332 | 8.3 |
| Ignored | 974 | 24.5 |
| Shared needles | ||
| No | 1596 | 40.1 |
| Yes | 2387 | 59.9 |
| Tattoos | ||
| No | 3852 | 96.7 |
| Yes | 131 | 3.3 |
| Piercings | ||
| No | 3952 | 99.2 |
| Yes | 31 | 0.8 |
| Acupuncture | ||
| No | 3974 | 99.8 |
| Yes | 9 | 0.2 |
| Sex life | ||
| Has never had sex | 1607 | 40.3 |
| Active | 1887 | 47.4 |
| Inactive | 489 | 12.3 |
| Condom use | ||
| Always | 459 | 11.5 |
| Sometimes | 743 | 18.7 |
| Never | 933 | 23.4 |
| Ignored | 241 | 6.0 |
| Does not apply | 1607 | 40.3 |
| Number of partners | ||
| Only 1 | 1752 | 44 |
| More than 1 | 243 | 6.1 |
| Ignored | 381 | 9.6 |
| Does not apply | 1607 | 40.3 |
| Promiscuity (more than two partners in six months) | ||
| No | 1638 | 41.1 |
| Yes | 209 | 5.2 |
| Ignored | 170 | 4.3 |
| Does not apply | 1966 | 49.4 |
| Number of Hepatitis B vaccine doses | ||
| 3 doses | 1047 | 26.3 |
| 2 doses | 147 | 3.7 |
| 1 dose | 117 | 2.9 |
| 0 dose | 1338 | 33.6 |
| Does not know | 1334 | 33.5 |
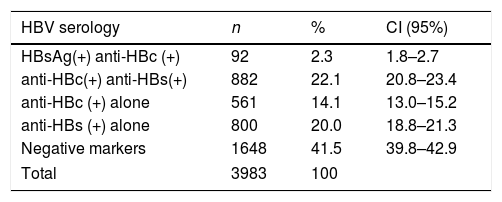
Prevalence: HBsAg, anti-HBc and anti-HBs prevalence rates are described in Table 2. Among the 92 HBsAg carriers, three (3.26%) exhibited atypical serological profiles (coexistence of HBsAg and anti-HBs) and have been described in a previous paper [13]. The overall prevalence of anti-HBc was 38.5% (95% CI: 37.0–40.0). The studied municipalities exhibited differences in prevalence: Axixá=17.5% (95% CI: 14.4–20.9), Morros=44.8% (95% CI: 41.4–48.3), Icatu=40.3% (95% CI 36.6–44.1), Humberto de Campos=43.1% (95% CI: 40.2–46.1) and Urbano Santos=38.8% (95% CI: 35.1–42.6).
Profile of HBV hepatitis serum markers. Maranhão State, Brazil, 2012–2016 (n=3983).
| HBV serology | n | % | CI (95%) |
|---|---|---|---|
| HBsAg(+) anti-HBc (+) | 92 | 2.3 | 1.8–2.7 |
| anti-HBc(+) anti-HBs(+) | 882 | 22.1 | 20.8–23.4 |
| anti-HBc (+) alone | 561 | 14.1 | 13.0–15.2 |
| anti-HBs (+) alone | 800 | 20.0 | 18.8–21.3 |
| Negative markers | 1648 | 41.5 | 39.8–42.9 |
| Total | 3983 | 100 | |
Among HBsAg carriers, eight were positive for anti-HDV (8.69%; 95% CI=2.90–14.40), among which four were from the municipality of Humberto de Campos, two from Morros and two from Urbano Santos.
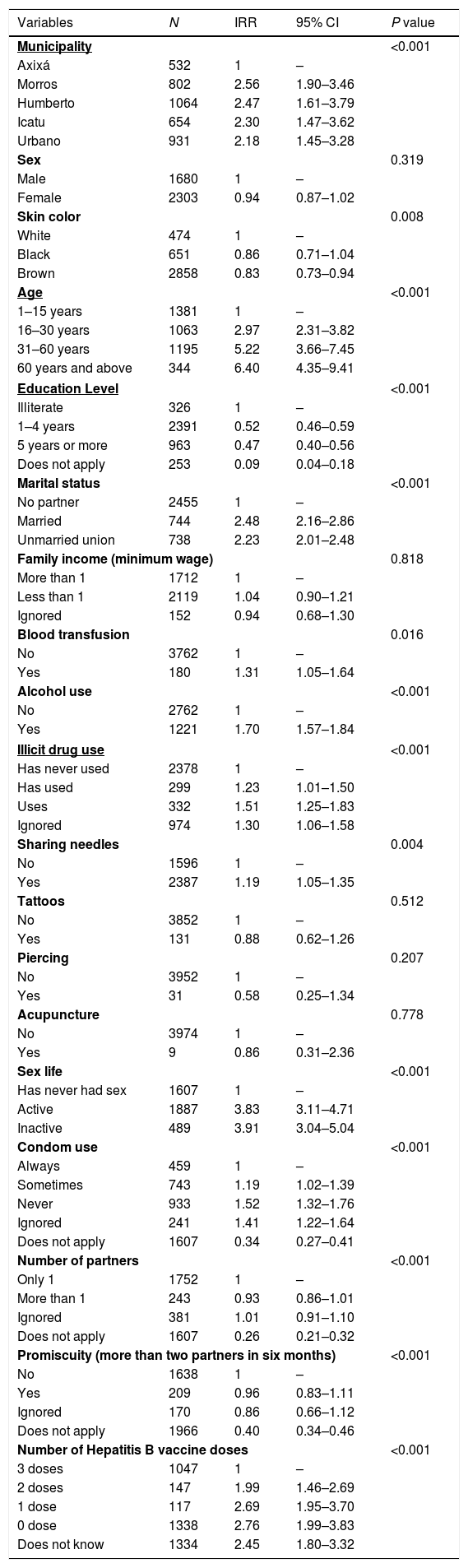
Table 3 exhibits the results of non-fitted analysis between the studied variables and positive anti-HBc, with the respective incidence-rate ratios (IRRs) and 95% CIs. The reference category was the one with the lowest transmission risk, and variables with P<0.2 were selected for the fitted model.
Non-fitted analysis of factors associated with HBV infection (anti-HBc-positive). Maranhão State, Brazil, 2012–2016 (n=3983).
| Variables | N | IRR | 95% CI | P value |
|---|---|---|---|---|
| Municipality | <0.001 | |||
| Axixá | 532 | 1 | – | |
| Morros | 802 | 2.56 | 1.90–3.46 | |
| Humberto | 1064 | 2.47 | 1.61–3.79 | |
| Icatu | 654 | 2.30 | 1.47–3.62 | |
| Urbano | 931 | 2.18 | 1.45–3.28 | |
| Sex | 0.319 | |||
| Male | 1680 | 1 | – | |
| Female | 2303 | 0.94 | 0.87–1.02 | |
| Skin color | 0.008 | |||
| White | 474 | 1 | – | |
| Black | 651 | 0.86 | 0.71–1.04 | |
| Brown | 2858 | 0.83 | 0.73–0.94 | |
| Age | <0.001 | |||
| 1–15 years | 1381 | 1 | – | |
| 16–30 years | 1063 | 2.97 | 2.31–3.82 | |
| 31–60 years | 1195 | 5.22 | 3.66–7.45 | |
| 60 years and above | 344 | 6.40 | 4.35–9.41 | |
| Education Level | <0.001 | |||
| Illiterate | 326 | 1 | – | |
| 1–4 years | 2391 | 0.52 | 0.46–0.59 | |
| 5 years or more | 963 | 0.47 | 0.40–0.56 | |
| Does not apply | 253 | 0.09 | 0.04–0.18 | |
| Marital status | <0.001 | |||
| No partner | 2455 | 1 | – | |
| Married | 744 | 2.48 | 2.16–2.86 | |
| Unmarried union | 738 | 2.23 | 2.01–2.48 | |
| Family income (minimum wage) | 0.818 | |||
| More than 1 | 1712 | 1 | – | |
| Less than 1 | 2119 | 1.04 | 0.90–1.21 | |
| Ignored | 152 | 0.94 | 0.68–1.30 | |
| Blood transfusion | 0.016 | |||
| No | 3762 | 1 | – | |
| Yes | 180 | 1.31 | 1.05–1.64 | |
| Alcohol use | <0.001 | |||
| No | 2762 | 1 | – | |
| Yes | 1221 | 1.70 | 1.57–1.84 | |
| Illicit drug use | <0.001 | |||
| Has never used | 2378 | 1 | – | |
| Has used | 299 | 1.23 | 1.01–1.50 | |
| Uses | 332 | 1.51 | 1.25–1.83 | |
| Ignored | 974 | 1.30 | 1.06–1.58 | |
| Sharing needles | 0.004 | |||
| No | 1596 | 1 | – | |
| Yes | 2387 | 1.19 | 1.05–1.35 | |
| Tattoos | 0.512 | |||
| No | 3852 | 1 | – | |
| Yes | 131 | 0.88 | 0.62–1.26 | |
| Piercing | 0.207 | |||
| No | 3952 | 1 | – | |
| Yes | 31 | 0.58 | 0.25–1.34 | |
| Acupuncture | 0.778 | |||
| No | 3974 | 1 | – | |
| Yes | 9 | 0.86 | 0.31–2.36 | |
| Sex life | <0.001 | |||
| Has never had sex | 1607 | 1 | – | |
| Active | 1887 | 3.83 | 3.11–4.71 | |
| Inactive | 489 | 3.91 | 3.04–5.04 | |
| Condom use | <0.001 | |||
| Always | 459 | 1 | – | |
| Sometimes | 743 | 1.19 | 1.02–1.39 | |
| Never | 933 | 1.52 | 1.32–1.76 | |
| Ignored | 241 | 1.41 | 1.22–1.64 | |
| Does not apply | 1607 | 0.34 | 0.27–0.41 | |
| Number of partners | <0.001 | |||
| Only 1 | 1752 | 1 | – | |
| More than 1 | 243 | 0.93 | 0.86–1.01 | |
| Ignored | 381 | 1.01 | 0.91–1.10 | |
| Does not apply | 1607 | 0.26 | 0.21–0.32 | |
| Promiscuity (more than two partners in six months) | <0.001 | |||
| No | 1638 | 1 | – | |
| Yes | 209 | 0.96 | 0.83–1.11 | |
| Ignored | 170 | 0.86 | 0.66–1.12 | |
| Does not apply | 1966 | 0.40 | 0.34–0.46 | |
| Number of Hepatitis B vaccine doses | <0.001 | |||
| 3 doses | 1047 | 1 | – | |
| 2 doses | 147 | 1.99 | 1.46–2.69 | |
| 1 dose | 117 | 2.69 | 1.95–3.70 | |
| 0 dose | 1338 | 2.76 | 1.99–3.83 | |
| Does not know | 1334 | 2.45 | 1.80–3.32 | |
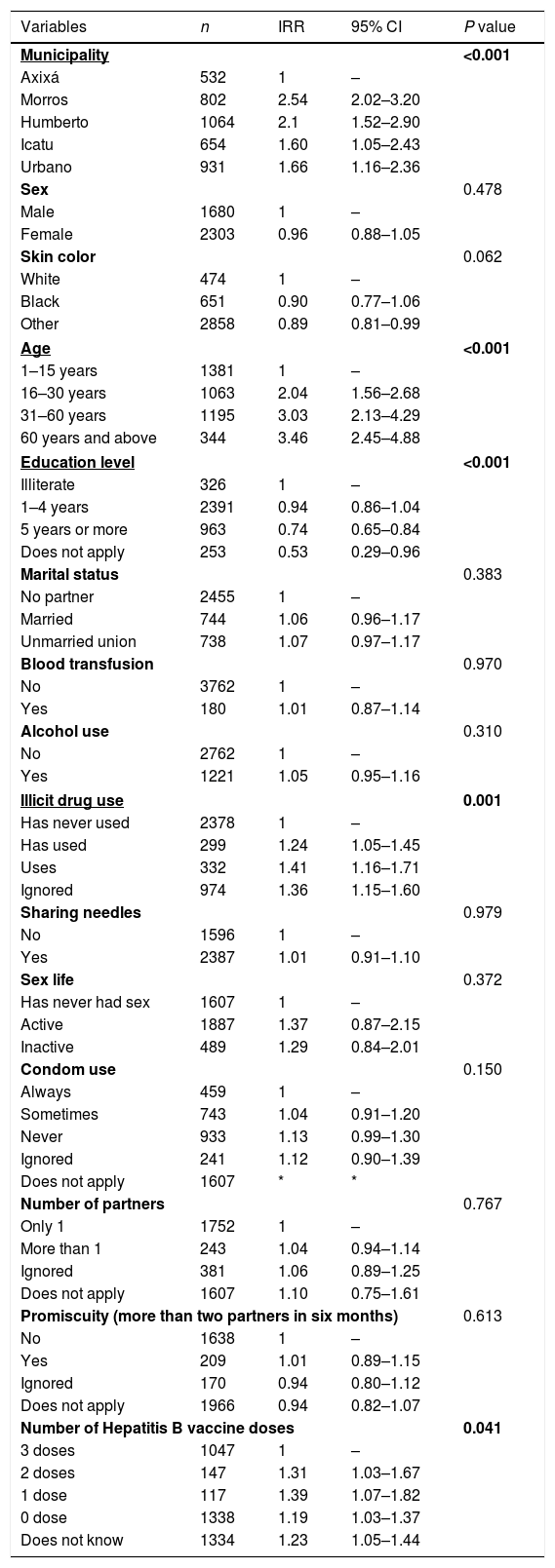
Table 4 shows the results of multivariate analysis. Compared to Axixá, the municipality of Morros exhibited a two-fold higher risk of contact for anti-HBc (IRR=2.54, 95% CI=2.02–3.20), followed by Humberto de Campos (IRR=2.1, 95% CI=1.52–2.90), Urbano Santos (IRR=1.66, 95% CI=1.16–2.36) and Icatu (IRR=16, 95% CI=1.05–2.43). Age was the associated factor, and an age above 60 years increased the risk three-fold (IRR=3.03, 95% CI=2.13–4.29). Education was also associated, and five or more years of schooling exhibited higher protection against infection (IRR=0.74, 95% CI=0.65–0.84). Illicit drug use (IRR=1.36, 95% CI=1.15–1.60) and incomplete vaccination were also associated with contact with HBV.
Factors associated with HBV (anti-HBc-positive), multivariate analysis. Maranhão State, Brazil, 2012–2016 (n=3983).
| Variables | n | IRR | 95% CI | P value |
|---|---|---|---|---|
| Municipality | <0.001 | |||
| Axixá | 532 | 1 | – | |
| Morros | 802 | 2.54 | 2.02–3.20 | |
| Humberto | 1064 | 2.1 | 1.52–2.90 | |
| Icatu | 654 | 1.60 | 1.05–2.43 | |
| Urbano | 931 | 1.66 | 1.16–2.36 | |
| Sex | 0.478 | |||
| Male | 1680 | 1 | – | |
| Female | 2303 | 0.96 | 0.88–1.05 | |
| Skin color | 0.062 | |||
| White | 474 | 1 | – | |
| Black | 651 | 0.90 | 0.77–1.06 | |
| Other | 2858 | 0.89 | 0.81–0.99 | |
| Age | <0.001 | |||
| 1–15 years | 1381 | 1 | – | |
| 16–30 years | 1063 | 2.04 | 1.56–2.68 | |
| 31–60 years | 1195 | 3.03 | 2.13–4.29 | |
| 60 years and above | 344 | 3.46 | 2.45–4.88 | |
| Education level | <0.001 | |||
| Illiterate | 326 | 1 | – | |
| 1–4 years | 2391 | 0.94 | 0.86–1.04 | |
| 5 years or more | 963 | 0.74 | 0.65–0.84 | |
| Does not apply | 253 | 0.53 | 0.29–0.96 | |
| Marital status | 0.383 | |||
| No partner | 2455 | 1 | – | |
| Married | 744 | 1.06 | 0.96–1.17 | |
| Unmarried union | 738 | 1.07 | 0.97–1.17 | |
| Blood transfusion | 0.970 | |||
| No | 3762 | 1 | – | |
| Yes | 180 | 1.01 | 0.87–1.14 | |
| Alcohol use | 0.310 | |||
| No | 2762 | 1 | – | |
| Yes | 1221 | 1.05 | 0.95–1.16 | |
| Illicit drug use | 0.001 | |||
| Has never used | 2378 | 1 | – | |
| Has used | 299 | 1.24 | 1.05–1.45 | |
| Uses | 332 | 1.41 | 1.16–1.71 | |
| Ignored | 974 | 1.36 | 1.15–1.60 | |
| Sharing needles | 0.979 | |||
| No | 1596 | 1 | – | |
| Yes | 2387 | 1.01 | 0.91–1.10 | |
| Sex life | 0.372 | |||
| Has never had sex | 1607 | 1 | – | |
| Active | 1887 | 1.37 | 0.87–2.15 | |
| Inactive | 489 | 1.29 | 0.84–2.01 | |
| Condom use | 0.150 | |||
| Always | 459 | 1 | – | |
| Sometimes | 743 | 1.04 | 0.91–1.20 | |
| Never | 933 | 1.13 | 0.99–1.30 | |
| Ignored | 241 | 1.12 | 0.90–1.39 | |
| Does not apply | 1607 | * | * | |
| Number of partners | 0.767 | |||
| Only 1 | 1752 | 1 | – | |
| More than 1 | 243 | 1.04 | 0.94–1.14 | |
| Ignored | 381 | 1.06 | 0.89–1.25 | |
| Does not apply | 1607 | 1.10 | 0.75–1.61 | |
| Promiscuity (more than two partners in six months) | 0.613 | |||
| No | 1638 | 1 | – | |
| Yes | 209 | 1.01 | 0.89–1.15 | |
| Ignored | 170 | 0.94 | 0.80–1.12 | |
| Does not apply | 1966 | 0.94 | 0.82–1.07 | |
| Number of Hepatitis B vaccine doses | 0.041 | |||
| 3 doses | 1047 | 1 | – | |
| 2 doses | 147 | 1.31 | 1.03–1.67 | |
| 1 dose | 117 | 1.39 | 1.07–1.82 | |
| 0 dose | 1338 | 1.19 | 1.03–1.37 | |
| Does not know | 1334 | 1.23 | 1.05–1.44 | |
The present study, which involved five municipalities of Maranhão State (Northeastern Brazil) and included individuals aged one year and above, identified a 2.3% (95% CI 1.8–2.7) seroprevalence of HBsAg, and eight (8.69%; 95% CI 2.90–14.40) of the HBV carriers were also seropositive for anti-HDV. These results confirmed the notions that the endemicity of HBV in the studied region was higher than expected for the Northeast region of Brazil (0.37%) and that there was, in fact, evidence of a significant presence of HDV.
The seroprevalence of HBsAg found in the present work is different from the current concept that Brazil has low endemicity for HBV (0.65%), according to the last systematic review of papers published between 1965 and 2013 [13], but confirms the information that Brazil still exhibits regions with endemicity above 2%, as reported by Souto (2016) in a recent systematic review of 100 Brazilian studies [14]. This prevalence applies especially to rural areas with precarious socioeconomic conditions, as is the case of the studied region of the present work. Specifically, in Maranhão State, a survey in a ‘quilombola’ community (descendants of African slaves) found an even higher seroprevalence of HBsAg (12.5%) [15].
In the evaluation of anti-HBc seropositivity (an indicator of the overall HBV infection rate), the sample exhibited a value of 38.5%, which is also above the means of both the nation and the Northeastern region, which are estimated at 11.6% and 11.7%, respectively [2,3], but is in agreement with the findings of a study from another municipality of the rural zone of Maranhão State (40.7%) [16], which is a different region from that studied in the present study. This finding suggests that Maranhão State is, in fact, a Brazilian state where HBV infection is a more significant aggravation than what is considered for most regions of the country because the results found here are equal to or above those of recent Brazilian studies on high-risk populations, including prison inmates, HIV-positive individuals or those with coagulopathies that require frequent blood transfusions [17–19].
Among the factors associated with higher HBV infection rates were the municipality of residence, older age, lower education level, history of illicit drug use, and absence of vaccines or incomplete vaccinations.
Living in the municipalities of Icatu, Humberto de Campos, Morros or Urbano Santos was independently associated with a higher risk of HBV infection compared with the municipality of Axixá. Even though these municipalities are contiguous, Axixá is classified as having a medium municipal human development index (HDI), whereas the remaining studied municipalities have low municipal HDIs (Brazilian Institute of Geography and Statistics; Instituto Brasileiro de Geografia e Estatística – IBGE). Lower socioeconomic indices have been associated with higher prevalence of HBV infection, especially when associated with the possibility of horizontal transmission due to inadequate habits of hygiene and disease prevention [20,21], which could explain the results found in the studied region of the present work.
In the same line of reasoning as the above, a higher education level, an important indirect indicator of socioeconomic development, was independently associated with a lower risk of HBV infection. These results have been observed in other national surveys [2,3,22,23] and even in developed countries, such as Italy [24], which further supports the concept that education level is a protective factor for specific infecto-contagious diseases because it is associated with better understanding of risky sexual behaviors, improving the prevention of sexually transmitted diseases (STDs) [25]. It is important to note that a lower education level is directly associated with lower awareness of harboring chronic HBV infection [26] and with a lower vaccination rate [27], thus perpetuating horizontal and vertical virus transmission.
Older age, both here and in other regions of the world [1–3,24], has been associated with HBV infection, thus representing higher chances of exposure to the virus, with sexual activity and with the fact that older individuals have not been submitted to vaccines, which, in Brazil, became compulsory in 1998.
Among the classical risk factors of infection, such as sexual behavior, use of sharp non-disposable materials, and history of blood transfusions [28], only illicit drug use was clearly associated with anti-HBc in this population. Another interesting finding is that most of the individuals who reported the above habit used marijuana or cocaine rather than injection drugs. Thus, this behavior might be associated with mechanisms of transmission that could not be clearly identified here, suggesting that these individuals must be prioritized for prevention.
In the present work, with participants aged one year and above (many with a complete vaccination schedule), we chose to include data on vaccination (yes or no) and the number of registered doses due to the lack of information in the country regarding the effect of the vaccine on the prevention of hepatitis B in the general population. The universal vaccination initiated during the first year of life in Brazil as of 1998 already included populations at risk and was progressively extended until, in 2015, all residents of the country were being covered [29]. As expected, the higher the number of vaccine doses the individual was submitted to, the lower the frequency of anti-HBc was. However, among those aged 30 years and above (1539 individuals), only 15% exhibited positivity for anti-HBs alone during data collection, thus demonstrating low vaccination coverage in this age range (a paper addressing the evaluation of the results of vaccination is being elaborated).
One of the most important results of the present study was the confirmation that HDV infection is a reality in Maranhão State since eight of the 92 HBsAg carriers were positive for anti-HDV. Previously published studies from this research describe the HDV and HBV genotypes identified among these 92 HBV carriers, which were confirmed as HDV-8 and HBV-D4 [30,31], as had been suspected. Since there were few HDV cases, the factors associated with infection could not be identified. However, cases were identified in the municipalities of Morros, Humberto de Campos and Urbano Santos. The prevalence of 8.6% in the present study was slightly above that found in Western Europe [32] but was not as high as that described in some African and Asian countries [10,33] and even in the Brazilian Amazon [34]. However, this is one of the few studies with a large sample of the general population of the country outside the Amazon and shows that HDV is a complicating factor that is associated with all of the others identified in Brazil, thus justifying the efforts in which the country is engaged, following the strategy of the WHO to combat viral hepatitis, which is a major global health problem [1], as emphasized in the last World Hepatitis Summit [35].
Of note, the present work has a very representative sample of the population and shows intermediate endemicity of HBV related with living conditions of the population and with the lack of robust preventive measures.
The present work has limitations and strengths. The main strength is the representative sample of these municipalities, given that the main sample was calculated using a prevalence of 0.5% for HBsAg, which is almost one-fifth of the real prevalence, thus allowing a safe analysis of the prevalence and associated factors. Among the limitations are those related to cross-sectional studies and a possible bias of information on sexual behavior and drug use because information was collected via interviews during a single home visit. Precautions, such as training the interviewers and performing individual interviews, were taken in an attempt to reduce these limitations, although they might not have been sufficient to avoid distortions.
5ConclusionThe findings of the present survey reinforce the heterogeneity of prevalence outside the Brazilian capitals and suggest factors that are associated with HBV transmission, showing the need to improve the use of high-impact strategies already regulated in the country, such as the immunization of children, adolescents and adults, in addition to strategies for the screening of infected individuals and for the prevention of vertical transmission, considering the implementation of new policies for populations at risk, such as illicit drug users. Our results also reinforce the need for further research to determine the real prevalence of HDV infection in the country.
AbbreviationsHBV hepatitis B Delta viral hepatitis B virus surface antigen anti-hepatitis B core antigen antibodies against hepatitis Delta virus
All authors made an intellectual contribution to the research contribution to research taking responsibility for the data and conclusions and approving the manuscript.
FundingThis work was supported by Fundação de Amparo à Pesquisa do Estado do Maranhão – (FAPEMA) [grant: PPSUS-03348/13 and PPP-01263/12]; Brazilian Ministry of Health [grant: 1/2013].
Conflict of interestNone.