Long thought to be hypocoagulable, new evidence suggests cirrhosis patients have “rebalanced” coagulation in the setting of decreased synthesis of both pro- and anti-coagulant factors. Traditional testing like PT/INR reflects only the decreased synthesis of pro-coagulant factors and thus does not correspond to bleeding or clotting risk in this population. In this review, we discuss the use of viscoelastic testing (VET), an assay of global hemostasis in cirrhosis patients. We describe the technique and interpretation of commercially available VET and assess the application of VET in both transplant and non-transplant cirrhosis populations. VET largely correlates well with traditional testing including platelet count and fibrinogen level, however, is potentially less accurate in patients with low fibrinogen levels. VET may be useful in identifying patients at higher risk of hypercoagulable complications post-transplant and reflects changes in hemostasis in decompensated patients. While VET has been associated with decreased transfusion support in multiple studies, the lack of bleeding in patients who avoided prophylactic transfusion suggests a “rescue” rather than prophylactic approach to transfusion may be ideal and further studies with a “rescue” arm are needed. Additional prospective studies of VET should include clinically relevant endpoints of bleeding and thrombosis.
Our understanding of coagulopathy in patients with chronic liver disease has evolved over the last decade.1,2 Old dogma suggested that patients with cirrhosis were “auto-anticoagulated.” This was supported by the prolonged prothrombin time (PT) and frequent bleeding episodes seen in patients with cirrhosis. Two broad clinical observations have since discredited this doctrine. First, despite abnormal coagulation testing, hospitalized patients with cirrhosis develop venous thrombosis at a rate at least comparable to patients without cirrhosis.3,4 Second, the risk of spontaneous or procedure-related bleeding is not predicted by abnormal traditional coagulation testing.5–8 Given these observations, cirrhosis patients are now believed to have a “rebalanced” coagulation system. This has been supported by recent studies showing normal thrombin generation potential in cirrhosis subjects compared to healthy controls.9,10 This new balance is precarious, however, as it is driven by decreased synthesis of liver derived pro- and anti-coagulant factors so that the resiliency of the hemostatic system is diminished in cirrhosis patients. The balance is disrupted when patients develop acute clinical conditions (e.g. infection, renal failure), placing them at risk of thrombotic or bleeding events.11–15
Given our new understanding of coagulation in liver disease patients, there is significant interest in other tests of coagulation that could provide clinicians with a truly global picture of the coagulation system in cirrhosis. PT and activated partial thromboplastin time (aPTT) are misleading in this patient population as they measure only the decreased synthesis of pro-coagulant factors. Furthermore, these tests are performed on plasma rather than whole blood and thus do not reflect endothelial tissue factor, blood flow, platelet function, and other factors that contribute to clot formation in vivo. Thrombin generation assays (TGA) have potential value in this population given TGA reflects activity of both pro- and anti- coagulant factors, but, similar to PT and aPTT, TGA is also performed on plasma rather than whole blood. Furthermore, TGA is relatively time-consuming and reagents are not yet standardized, limiting its clinical utility at this point in cirrhosis patients.
Viscoelastic testing (VET) is a promising technique for evaluation of hemostasis. VET measures clot strength as a reflection of the shear strength of clot formation and dissolution in whole blood. It is performed on whole blood assessing coagulation in a more global and potentially more clinically relevant fashion. VET offers another advantage to the clinician in that it can be performed relatively rapidly, in minutes, and is thus useful to guide hemostatic therapy in bleeding patients. This review will describe the application of VET in cirrhosis patients.
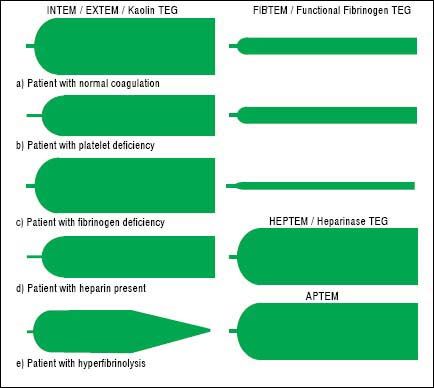
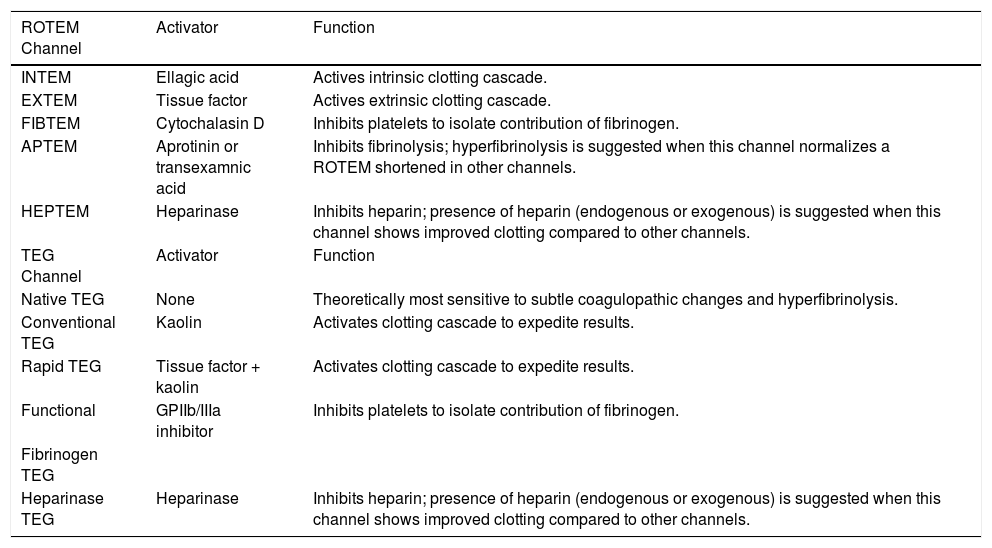
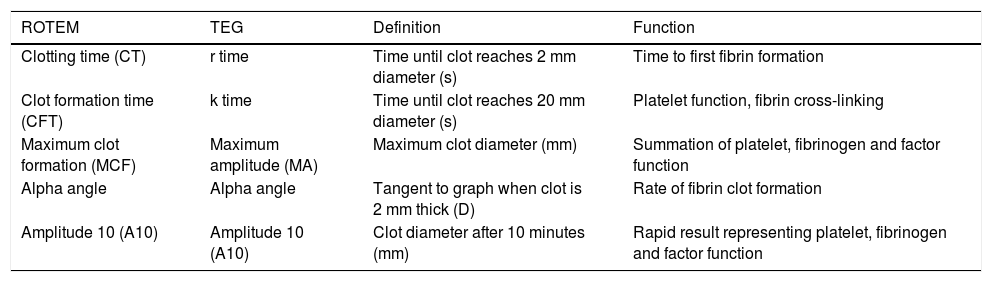
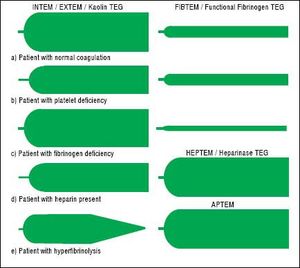
TechniqueFirst developed in 1948, VET has been used to guide transfusion support during liver transplant since 1985.16,17 Outside of liver disease, VET is utilized frequently in the care of surgical patients at high risk of bleeding and thrombosis. Both cardiothoracic and trauma surgery patients, for example, are often monitored intraoperatively with VET.18,19 The two most widely used commercially available assays are thromboelastography (TEG) and rotational thromboelastometry (ROTEM). In TEG, whole blood is rotated in a cup that contains a suspended pin. In ROTEM, whole blood in held in a stationary cup with a central rotating pin.20,21 In each, the strength of clot formation and dissolution is reflected by a graphic tracing generated from the resistance of the blood to rotation (Figure 1). In TEG, this is monitored from a tracing wire affixed to the suspended pin, whereas ROTEM relies on an optical detection system. In each assay, various activators can be added to the blood to better assess different portions of clotting cascade (Table 1). Of note, activators that reduce test turnaround time (eg. kaolin) could theoretically blunt the sensitivity of VET to subtle changes in coagulation and clot lysis. The graphical tracings of both TEG and ROTEM are quantified with standardized measurements (Table 2).
In addition to TEG and ROTEM, sonorheometry, a type of VET that relies on the measurement of ultrasound pulses through clotting blood rather than mechanical shearing as in ROTEM and TEG, is also being investigated.22 While the avoidance of mechanical shear forces is appealing, there are not yet published data on the use of sonorheometry in liver disease.
Given the similarities in principal of TEG and ROTEM, choice of methodology is typically dependent on local expertise. One study compared both TEG and ROTEM prospectively in liver transplant patients and suggested INTEM CT and EXTEM CT were more sensitive than kaolin and rapid TEG for detection of factor deficiencies in patients undergoing liver transplant.23 In non-cirrhosis populations, a prospective study of patients undergoing elective cardiac surgery compared TEG to ROTEM to evaluate both inter- and intra-operator variability. They found the variation coefficient was lower for ROTEM than TEG and attributed this to the automated pipetting feature of ROTEM.24
InterpretationAs illustrated in tables 1 and 2, both ROTEM and TEG return a battery of results to the ordering clinician, pro-viding a level of information that can be daunting. Classically, tracings are interpreted qualitatively based on changes to the shape of the curve as illustrated in figure 1. The subjective nature of visual interpretation can lead to inter-observer variability and thus there has been interest in correlating specific ROTEM and TEG findings with traditional transfusion parameters.
Activators and functionalities of available rotational thromboelastometry (ROTEM) and thrombelastography (TEG) channels.
| ROTEM Channel | Activator | Function |
|---|---|---|
| INTEM | Ellagic acid | Actives intrinsic clotting cascade. |
| EXTEM | Tissue factor | Actives extrinsic clotting cascade. |
| FIBTEM | Cytochalasin D | Inhibits platelets to isolate contribution of fibrinogen. |
| APTEM | Aprotinin or transexamnic acid | Inhibits fibrinolysis; hyperfibrinolysis is suggested when this channel normalizes a ROTEM shortened in other channels. |
| HEPTEM | Heparinase | Inhibits heparin; presence of heparin (endogenous or exogenous) is suggested when this channel shows improved clotting compared to other channels. |
| TEG Channel | Activator | Function |
| Native TEG | None | Theoretically most sensitive to subtle coagulopathic changes and hyperfibrinolysis. |
| Conventional TEG | Kaolin | Activates clotting cascade to expedite results. |
| Rapid TEG | Tissue factor + kaolin | Activates clotting cascade to expedite results. |
| Functional | GPIIb/IIIa inhibitor | Inhibits platelets to isolate contribution of fibrinogen. |
| Fibrinogen TEG | ||
| Heparinase TEG | Heparinase | Inhibits heparin; presence of heparin (endogenous or exogenous) is suggested when this channel shows improved clotting compared to other channels. |
Common parameters used to quantify results from rotational thromboelastometry (ROTEM) and thromboelastography (TEG).
| ROTEM | TEG | Definition | Function |
|---|---|---|---|
| Clotting time (CT) | r time | Time until clot reaches 2 mm diameter (s) | Time to first fibrin formation |
| Clot formation time (CFT) | k time | Time until clot reaches 20 mm diameter (s) | Platelet function, fibrin cross-linking |
| Maximum clot formation (MCF) | Maximum amplitude (MA) | Maximum clot diameter (mm) | Summation of platelet, fibrinogen and factor function |
| Alpha angle | Alpha angle | Tangent to graph when clot is 2 mm thick (D) | Rate of fibrin clot formation |
| Amplitude 10 (A10) | Amplitude 10 (A10) | Clot diameter after 10 minutes (mm) | Rapid result representing platelet, fibrinogen and factor function |
For the clinician, the single most useful VET parameter is the final measure of clot stiffness, reflected as the maximum clot formation (MCF) in ROTEM or maximum amplitude (MA) in TEG, as this parameter represents the summation of primary and secondary hemostasis. In the ROTEM EXTEM or INTEM channels and on a kaolin TEG, this largely reflects platelet contribution. In ROTEM FIBTEM or functional fibrinogen TEG (ffTEG), both performed in the presence of an anti-platelet agent, MCF/MA size is driven by fibrinogen and fibrin clot strength.25 INTEM MCF and EXTEM MCF are largely interchangeable and an EXTEM MCF of 45 mm correlates with a platelet count of at least 52 × 103/mL while a FIBTEM MCF of 8 mm correlates with a fibrinogen level of at least 128 mg/dL.26 In studies using VET to guide transfusion, TEG MA from 30 to 55 mm and EXTEM MCF of 40 mm have been used to trigger platelet transfusion while a FIBTEM MCF of < 8 mm has been used as a cutoff for cryoprecipitate transfusion.27–30 While there are multiple studies correlating VET with traditional testing, there are scant data available demonstrating the relationship of specific VET parameters with bleeding or clotting events.
Viscoelastic Testing in Liver TransplantationNumerous studies have been performed evaluating the use of ROTEM and TEG in liver transplantation.23,25–29,31–39The appropriate threshold on ROTEM and TEG to initiate transfusion27–29,36 has been studied, as well as the correlation of VET with traditional testing and use of different techniques to reduce result turn around time.25,26,29,31–33 The utility of TEG in predicting hypercoagulable patients has been examined34,37,38 as well as the ability of TEG and ROTEM to assess hyperfibrinolysis.35,36,39
Correlation with Traditional TestingRotemFor studies correlating ROTEM with traditional coagulation testing, focus has largely been on correlating MCF and amplitude 10 (A10) or amplitude 5 (A5) values with traditional testing. Two recent studies found good correlation of INTEM, EXTEM and FIBTEM MCF with platelet count and fibrinogen level in liver transplantation.25,26 Of note, at low levels of fibrinogen (< 100 mg/dL), FIBTEM MCF correlated less well, making it less reliable in this group.25 Multiple groups have also evaluated the correlation between A5 and A10 with traditional testing in an attempt to reduce test turnaround time.29,31,32 Both A5 and A10 correlate well with MCF values in all channels and are predictive of platelet count and fibrinogen level. An EX-TEM A10 of 27 to 35 mm was found to reflect a platelet count of 50 × 103/mL and a FIBTEM A10 of 5 corresponded with a fibrinogen level of < 100 mg/dL.29,31,32
TegThere are relatively few studies for correlation of TEG with traditional coagulation testing. One study prospectively evaluated rapid TEG versus kaolin TEG and functional fibrinogen TEG versus fibrinogen level in liver transplant patients.33 Rapid TEG MA correlated well with kaolin TEG MA and reduced the average time to measure MA. Functional fibrinogen TEG correlated well with fibrinogen level on measurements obtained at time of skin incision. This correlation weakened, however, for samples collected thirty minutes after graft reperfusion at which point samples on average had significantly lower fibrinogen levels, echoing the ROTEM data.
A second study evaluated TEG in cirrhosis subjects undergoing liver transplantation versus healthy controls.34 Subjects with cirrhosis tended to have a more hypocoagulable profile than controls overall, however, interestingly, there was a subset of cirrhosis subjects (9.5%) with a shorter than normal r time compared to healthy controls, suggesting faster clot activation. Further clinical characterization of this subset of patients was not provided. Finally, an additional prospective study evaluated the utility of PT/ international normalized ratio (INR), TEG and ROTEM in identifying factor deficiencies which were quantified by obtaining factor X activity.23 INR predicted factor deficiency well and ROTEM, particularly INTEM, was the best VET for predicting factor deficiency. The authors hypothesized that concomitant factor V deficiency may have explained the increased sensitivity of INTEM to severe factor deficiencies over kaolin TEG and EXTEM.
Transfusion GuidanceRotemVET has also been used as a parameter to guide transfusions in the setting of liver transplantation. Two studies used ROTEM and two, published by the same group, relied on TEG. The first ROTEM-based study performed a retrospective review of patients transfused based on current center guidelines using traditional measures of coagulation in an attempt to delineate ROTEM-based transfusion guidelines.29 In this study, an MCF of 40 mm on EXTEM and of 8 mm on FIBTEM were the most useful thresholds for transfusion of platelets and cryoprecipitate, respectively. The second ROTEM study prospectively compared ROTEM-based transfusion during liver transplantation to standard of care (SOC) transfusion protocols.36 The ROTEM-guided group in this study had a non-significant trend towards less red blood cell (RBC) and fresh frozen plasma (FFP) use. Of note, both groups received a significant amount of blood products with, on average, each patient in the ROTEM-guided group receiving 10 units of FFP vs. 13 units per patient in the SOC group.
TegWang, et al. evaluated the use of TEG to guide transfusions in liver transplant recipients in 2010 and 2012.27,28 The initial series prospectively compared the use of SOC transfusion guidelines to a group whose transfusions were guided by TEG. The TEG-guided group received significantly less FFP, an average of 12.8 units vs. 21.5 units, and had comparable intra-operative blood loss and red cell transfusion receipt. The second study retrospectively compared two different TEG transfusion thresholds, the initial TEG threshold used above versus a 35% higher threshold. Again a lower need for FFP as well as platelets was found with no evidence of increased bleeding events in the group who received less products. Given this data’s retrospective nature and the potential for improvements in surgical technique over time, these findings must be interpreted cautiously, but do suggest that TEG could provide supportive data to minimize unnecessary transfusions.
Evaluation Of HypercoagulabilityIn addition to evaluating VET for guidance in transfusion, some studies have evaluated the ability of VET to predict hypercoagulability. One group retrospectively reviewed TEG studies in liver transplant patients to compare TEG findings in patients with and without early hepatic artery thrombosis (HAT).37 Early HAT (within 21 days of transplantation) was seen in 2.7% of patients and late HAT was seen in 6.7% of patients. An MA of ≤ 65 mm on a preoperative kaolin TEG had a good negative predictive value for early HAT on multivariate analysis that included preoperative platelet count, re-transplant status, recipient body mass index, Roux-en-Y anastomosis and cryoprecipitate transfusion. The authors suggested that patients with a higher MA may benefit from more aggressive ultrasound surveillance and consideration of prophylactic anticoagulation.
An additional retrospective review of TEG in liver transplant recipients followed patients who developed a thrombotic complication within 30 days of transplant.38 Six patients (5%) undergoing primary transplantation developed HAT within 30 days of transplant. Of these 6 patients, 4 had shortened r times and 3 had an elevated G value (a mathematical calculation from MA to allow linear comparison) although neither of these was statistically significant. Patients with cholestatic diseases -primary biliary cholangitis and primary sclerosing cholangitis- tended to have higher G values that other diagnoses and 15.53% of patients overall had a high G value on kaolin TEG. Patients with cholestatic disease had increased clot stiffness by TEG in other studies as well.34 While relatively small, both studies evaluating HAT raise the question of closer monitoring and/or prophylactic anticoagulation in patients with hyper-coagulable-appearing TEG findings at time of transplant.37,38
Evaluation of HyperfibrinolysisHyperfibrinolysis is often a clinical diagnosis manifested by delayed procedural bleeding or diffuse mucosal bleeding. Currently diagnosis is clinical without specific laboratory tests to rely upon. Several studies have examined VET as a tool to identify hyperfibrinolysis. One group performed both TEG and ROTEM in liver transplant patients and evaluated the sensitivity of these tests to detect hyperfibrinolysis as defined by the manufacturer.35 Hyperfibrinolysis was confirmed by assay normalization in the APTEM channel. Two hundred and fifty samples were obtained from 376 patients and hyperfibrinolysis was identified in 89 (36%) of these samples. ROTEM was more sensitive than kaolin TEG for the detection of hyperfibrinolysis (p < 0.001). This difference was attributed to use of an intrinsic pathway activator (kaolin) in TEG versus the tissue factor used in EXTEM.
A second study evaluating the use of ROTEM to identify hyperfibrinolysis compared patients that had no laboratory monitoring for hyperfibrinolysis to a subsequent cohort of patients who had ROTEM monitoring.36 Hyperfibrinolysis, defined as maximum lysis > 15% on ROTEM, was found in 7.7% of patients. APTEM was performed, however, it is unclear whether all hyperfibrinolysis was confirmed with normalization of APTEM. Bleeding complications were also not reported, making the clinical significance of this finding uncertain. Finally, a small prospective pilot study of hyperfibrinolysis in six transplant patients showed no hyperfibrinolysis (defined as loss in clot amplitude > 5% 60 minutes after MA was reached on TEG) in any study patients.39 Given the small number of patients studied and variability of results, more investigation into the role of VET in identification of hyperfibrinolysis is needed.
Viscoelastic Testing in Chronic Liver DiseaseNumerous studies have evaluated the utility of TEG and ROTEM in chronic liver disease outside of liver transplantation.9–12,14,15,30,40–44 Many of these studies have evaluated VET alongside traditional coagulation testing and markers of prognosis in cirrhosis patients in an effort to delineate prognostic value and use of VET for identifying bleeding and clotting risk in this group.9,10,30,40–43 VET have also been used to characterize coagulation in chronic liver disease patients with acute complications including bleeding, portal venous thrombosis, infection, encephalopathy, kidney injury and acute liver failure.11–15
Correlation with Traditional TestingRotemIn a prospective study evaluating traditional coagulation testing and ROTEM in cirrhosis subjects and healthy controls, MCF and clot formation time (CFT) correlated well with platelet count and fibrinogen and had an AUC that allowed cirrhosis subjects to be distinguished from healthy controls.42 An additional study comparing FIBTEM in cirrhosis patients and healthy controls also found FIBTEM MCF correlated well with fibrinogen level and tended to decrease as liver disease advanced.43
A third study prospectively evaluated both ROTEM and TGA in samples from cirrhosis patients and healthy controls.10 While ROTEM appeared hypocoagulable in cirrhosis patients, TGA, even in the absence of thrombomodulin (TM), demonstrated increased endogenous thrombin potential and time to peak, suggesting hypercoagulability in this same group of cirrhosis subjects. Of note, subgroup analysis performed on patients who were noted to have a history of bleeding and/or clotting did not find observable differences in ROTEM or TGA in these groups.
These findings were similar to an additional study prospectively performing ROTEM and TGA in cirrhosis subjects.9 Again, while evidence of hypocoagulability in cirrhosis subjects was found on traditional testing and ROTEM, this was not supported by thrombin generation which, in the presence of TM, demonstrated preserved coagulation. INTEM and EXTEM MCF correlated well with platelet count and FIBTEM MCF correlated well with fibrinogen level. Potentially related to the addition of supraphysiologic amounts of TM in TGA, the disagreement between ROTEM and TGA in these studies warrants further exploration.
TegOne study comparing prospectively obtained TEG and traditional coagulation parameters in patients with cirrhosis, non-cirrhotic liver disease and healthy controls showed that kaolin TEG MA and CI correlated strongly with platelet count in all groups.40 Correlation of PT/INR with r and k times was weak as was correlation of TEG parameters with model for end stage liver disease (MELD) and Child Turcotte Pugh (CTP) scores. On TEG, nearly half of cirrhosis subjects had evidence of hypocoagulability, but, a small subset, 2.4%, appeared hypercoagulable.
A second study prospectively obtained TEG in hospi-talized (41% ICU, 59% medical ward), acutely ill cirrhosis subjects and healthy controls.41 Compared to healthy controls, cirrhosis subjects had a relatively hypocoagulable profile in kaolin, rapid, heparinase and functional fibrinogen TEGs. Interestingly, fibrinolysis was reduced in cirrhosis subjects as well compared to healthy controls on rapid TEG. Nearly half of the patients in this study received some degree of anticoagulation however this was not captured by TEG when they compared patients on and off of anticoagulation.
Transfusion GuidanceThere has been a single prospective trial to date evaluating the utility of VET in guiding transfusion in cirrhosis patients outside of the transplantation setting. In this study, cirrhosis patients with INR > 1.8 or platelet count < 50 × 103 / mL were randomized to TEG-guided transfusion vs. SOC transfusion support prior to undergoing invasive procedures.30 Patients underwent a variety of procedures which were both high and low risk for bleeding. One hundred percent of patients in the SOC group received transfusion of either FFP or platelets versus 16% of patients in the TEG-guided group. This difference was largely driven by FFP use with 53% of SOC group receiving FFP vs. 0% of TEG group. One patient in the SOC group had procedural-related bleeding after a paracentesis while there were no bleeding complications in patients in the TEG group. RBC transfusion was comparable in both groups and there was no survival difference at 90 days. While a reduction in transfusion in the TEG-guided group was demonstrated, SOC transfusion goals in this study were relatively aggressive, particularly the INR goal of 1.8, and may not reflect current practices. Furthermore the low rates of bleeding in both arms argue strongly for the addition of a control arm evaluating the outcome of no prophylactic transfusion prior to procedures.45
Vet in Acute IllnessSeveral studies have investigated the effect of acute changes in chronic liver disease patients by VET. Patients with early re-bleeding from esophageal varices have been noted to have appreciable differences from patients who did not re-bleed with longer r and k times and a more acute alpha angle suggestive of hypocoagulability.11 In this group, TEG MA and traditional coagulation tests were no different in re-bleeders and non-rebleeders. In the setting of bacterial infection, cirrhosis inpatients with documented infections were observed to have hypocoagulable changes in TEG.12 These changes improved in patients whose infection resolved and persisted in those patients whose infectious process lingered. Another study evalu-ating native and heparinase TEGs in cirrhosis patients with infection detected the presence of endogenous heparinoids in cirrhosis subjects with infection that was not present in uninfected controls, generating a potential mechanism for the observed hypocoagulability in infected subjects.15 Cirrhosis patients with PVT compared to those without PVT did not have a detectable difference on ROTEM parameters.14
There are scant data available regarding coagulopathy in patients with acute liver injury and failure. One study in which TEG was performed in non-cirrhosis patients with acute liver injury (ALI) and acute liver failure (ALF) found that despite elevated INR (mean 3.4 ± 1.7), 63% of patients had normal TEG parameters.13 In the ALF group, patients with clinical encephalopathy were noted to have a higher alpha angle and MA as well as shorter k time and were thought to be relatively hypercoagulable. Infected patients, patients with AKI and patients with bleeding or clotting events were noted to have long r times. No other TEG parameters were different in patients with acute complications compared to those without.
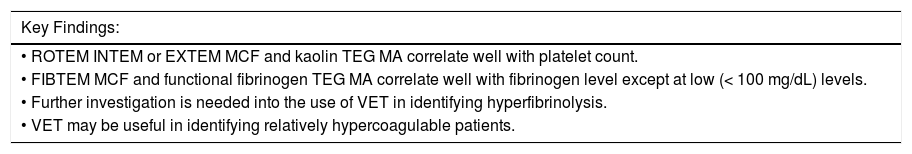
ConclusionKey findings are reviewed in table 3. VET correlates well with platelet and fibrinogen levels with the potential exception of patients with very low fibrinogen.25,31–33 VET was sensitive to hypercoagulability37,38 and may be useful in determining which patients are at higher risk of HAT after transplant. Small studies evaluating fibrinolysis were hindered by the lack of a reference ranges for cirrhosis subjects and lack of bleeding events in this group to support clinical relevance of these findings.10,34–36,41 VET identified dynamic changes in hemostasis during decompensation events11–13,15,35,44 and decreased the amount of transfusions given both in the setting of transplantation and invasive procedures.27–30,36 These data suggest that VET has utility as a rapid pointof-care test to guide transfusions in cirrhosis patients with bleeding and those undergoing transplantation. The lack of additional bleeding complications in patients who received less blood products when VET was used to guide transfusion27–30,36 calls into question the need for prophylactic transfusion in this patient population, particularly given the rebalancing of coagulation seen on both VET and TGA.9,10 Additional prospective studies are needed that include clinically relevant endpoints of bleeding and thrombosis in this group, ideally with a control group that receives rescue transfusions rather than prophylactic support.
Key results from data reviewed.
| Key Findings: |
|---|
| • ROTEM INTEM or EXTEM MCF and kaolin TEG MA correlate well with platelet count. |
| • FIBTEM MCF and functional fibrinogen TEG MA correlate well with fibrinogen level except at low (< 100 mg/dL) levels. |
| • Further investigation is needed into the use of VET in identifying hyperfibrinolysis. |
| • VET may be useful in identifying relatively hypercoagulable patients. |
ROTEM: rotational thromboelastometry. MCF: maximum clot firmness. MA: maximum amplitude. TEG: thromboelastography. VET: viscoelastic testing.
- •
A10: amplitude 10.
- •
A5: amplitude 5.
- •
ALF: acute liver failure.
- •
ALI: acute liver injury.
- •
aPTT: activated partial thromboplastin time.
- •
CFT: clot formation time.
- •
CTP: Child Turcotte Pugh.
- •
FFP: fresh frozen plasma.
- •
ffTEG: function fibrinogen TEG.
- •
HAT: hepatic artery thrombosis.
- •
INR: international normalized ratio.
- •
MA: maximum amplitude.
- •
MCF: maximum clot formation.
- •
MELD: model for end stage liver disease.
- •
PT: prothrombin time.
- •
RBC: red blood cell.
- •
ROTEM: rotational thrombelastometry.
- •
SOC: standard of care.
- •
TEG: thromboelastography.
- •
TGA: thrombin generation assays.
- •
TM: thrombomodulin.
- •
VET: viscoelastic testing.