As a fatal clinical syndrome, acute liver failure (ALF) is characterized by overwhelming liver inflammation and hepatic cell death. Finding new therapeutic methods has been a challenge in ALF research. VX-765 is a known pyroptosis inhibitor and has been reported to prevent damage in a variety of diseases by reducing inflammation. However, the role of VX-765 in ALF is still unclear.
Materials and MethodsALF model mice were treated with D-galactosamine (D-GalN) and lipopolysaccharide (LPS). LO2 cells were stimulated with LPS. Thirty subjects were enrolled in clinical experiments. The levels of inflammatory cytokines, pyroptosis-associated proteins and peroxisome proliferator-activated receptor α (PPARα) were detected using quantitative reverse transcription-polymerase chain reaction (qRT‒PCR), western blotting and immunohistochemistry. An automatic biochemical analyzer was used to determine the serum aminotransferase enzyme levels. Hematoxylin and eosin (HE) staining was used to observe the pathological features of the liver.
ResultsWith the progression of ALF, the expression levels of interleukin (IL) -1β, IL-18, caspase-1, and serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were increased. VX-765 could reduce the mortality rate of ALF mice, relieve liver pathological damage, and reduce inflammatory responses to protect against ALF. Further experiments showed that VX-765 could protect against ALF through PPARα, and this protective effect against ALF was reduced in the context of PPARα inhibition.
ConclusionsAs ALF progresses, inflammatory responses and pyroptosis deteriorate gradually. VX-765 can inhibit pyroptosis and reduce inflammatory responses to protect against ALF by upregulating PPARα expression, thus providing a possible therapeutic strategy for ALF.
Acute liver failure (ALF) is a clinical syndrome characterized by serious inflammatory-mediated hepatocellular injury with high mortality. Liver inflammation heavily affects ALF pathogenesis, and no effective treatment is available except for liver transplantation [1,2]. Studies have shown that systemic inflammatory responses are of vital importance in the outcome of ALF. Hepatocyte death in ALF is a result of the activation of systemic inflammatory responses generated by uncontrolled immune-mediated liver injury [3,4]. Although great progress has been made in ALF etiology and pathogenesis for the past few years, ALF is a serious and complex liver disease, and the immunological mechanism of organ damage is not fully understood.
VX-765 is a selective caspase-1 inhibitor that was shown to attenuate pyroptosis in a large number of studies [5–7]. The types of cell death from a molecular perspective include apoptosis, ferroptosis, pyroptosis, etc. As a new mode of cell death, pyroptosis is a kind of programmed lytic cell death closely related to inflammatory responses. During this process, caspase-1 is activated with the release of large amounts of proinflammatory cytokines such as IL-1β and IL-18 [8,9]. VX-765 can reduce inflammatory responses in vivo and in vitro, and a number of experiments have suggested that the NF-κB signaling pathway is closely related to this process [10,11]. Reportedly, VX-765 can prevent a variety of systemic diseases, such as traumatic brain injury, intestinal ischemia‒reperfusion injury and spinal cord injury. However, the role and pathological mechanism of VX-765 in the context of ALF are not clear.
PPARα, which is a nuclear transcription factor, has been confirmed to be involved in apoptosis, lipid metabolism and inflammatory responses [12–15]. Numerous studies have shown that PPARα has strong anti-inflammatory effects [16–19]. Our earlier studies revealed that PPARα could protect against ALF [20]. Thus, we hypothesize that the expression levels of proinflammatory cytokines and pyroptosis-related proteins are increased with the progression of ALF and that inhibiting pyroptosis with VX-765 can upregulate PPARα expression, relieve inflammatory responses and protect against ALF.
2Materials and Methods2.1Experimental miceWild-type C57BL/6 mice (male, 8-10 weeks) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and fed without specific pathogens in the animal facilities of Hebei Medical University. The ALF mouse model was induced by intraperitoneal injection of D-GalN (700 mg/kg; Sigma, St. Louis, MO, USA) and LPS (10 μg/kg; Sigma, St. Louis, MO, USA) or with normal saline in the control group. VX-765 (100 mg/kg; MCE, New Jersey, USA), which can inhibit the expression and activity of caspase-1, was intraperitoneally injected 2 hours prior to D-GalN/LPS treatment in ALF mice. VX-765 was dissolved in dimethyl sulfoxide (DMSO), and 1% DMSO equal to the treatment volume was injected into the control group. PPARα was suppressed by injecting PPARα siRNA (50 μM/kg, RIBOBIO, GUANGZHOU, China) through the tail vein 24 hours before D-GalN/LPS treatment, and the sequence was 5′-CTACAGAGACATGTACTGA-3′. All operations were performed with reference to the manufacturer's manuals. Six hours after D-GalN/LPS injection, the mice were euthanized, and serum samples and liver tissues were obtained for later analysis.
2.2Cell culture and treatmentHuman LO2 cells, which were a gift from the Hebei Medical University of China, were cultured in 1640 medium containing penicillin/streptomycin (1%) and fetal bovine serum (10%) in an incubator filled with 5% CO2 at 37°C. LO2 cells were exposed to LPS (20 ng/ml; Sigma) for 6 hours to induce inflammation. VX-765 (50 μM; MCE), which was dissolved in dimethyl sulfoxide (DMSO), was administered 2 hours before LPS. In the same way, 0.1% DMSO equivalent to the volume of the VX-765 treatment group was given to LO2 cells in the control group. PPARα inhibition was achieved by applying PPARα siRNA (50 nM, RIBOBIO, GUANGZHOU, China) 24 hours before LPS, and the sequence was 5′- GGAGCATTGAACATCGAAT-3′.
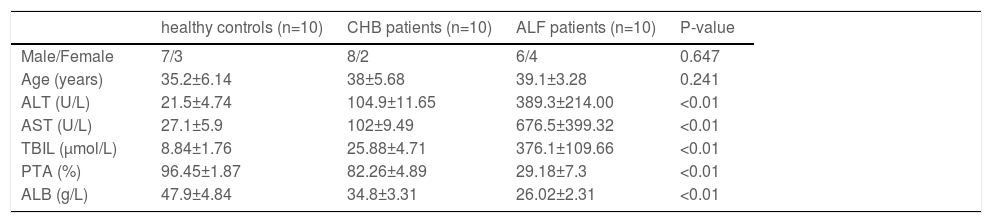
2.3Human specimensIn this study, liver samples in the ALF group were obtained from 10 liver transplantation patients. Chronic hepatitis B (CHB) samples were collected from 10 subjects who underwent liver puncture biopsy. Liver samples in the normal control group were obtained from 10 subjects who underwent hepatectomy during liver transplantation. A summary of the clinical characteristics of the subjects is presented in Table 1.
The clinical characteristics of the subjects enrolled in this study
ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; PTA, prothrombin time activity; ALB, albumin.
A multiparameter analyzer (LABOSPECT 008 AS, HITACHI, Japan) was used to examine the serum biochemical markers ALT and AST in liver injury according to the automatic program. The liver tissue was fixed in neutral formaldehyde solution at a concentration of 10%, embedded in paraffin and cut into 5 μm slices. After being dewaxed in xylene, the tissue was rehydrated with ethanol and stained with HE. Light microscopy was used to observe pathological characteristics of the liver.
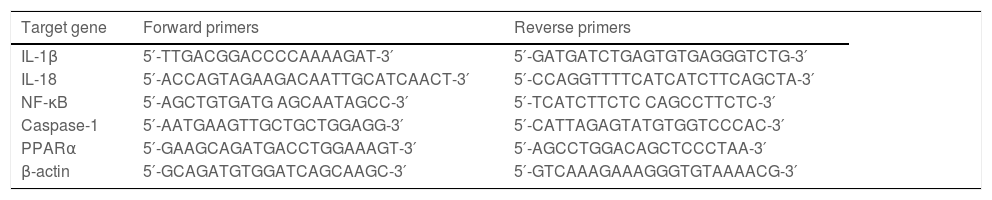
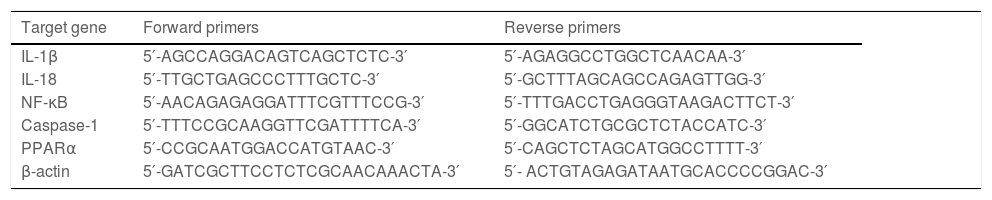
2.5Quantitative RT‒PCRTRIzol reagent was used to extract total RNA from hepatic tissue samples. According to the directions, RNA was reverse transcribed into cDNA with the PrimeScriptTM RT Reagent Kit (Takara, Tokyo, Japan). Quantitative RT‒PCR was performed using a SYBR Green RT‒PCR kit (Takara, Tokyo, Japan). The relative expression levels of the target genes were normalized to the level of β-actin, which was the reference gene. ΔΔCt was obtained as follows: ΔCttreated-ΔCtcontrol, and 2−ΔΔCt was used to represent the relative mRNA expression of target genes. The sequences of primers are presented in Table 2 and Table 3.
Primers for mouse genes
Primers for human genes
Proteins were extracted from hepatic tissue samples with radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors. The proteins were subjected to polyacrylamide gel electrophoresis and then transferred to polyvinylidine fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After being reacted with the primary antibody (1:200-2000) at 4°C overnight, the membranes were washed three times using Tris-buffered saline with Tween-20 (TBST) and then incubated with the indicated secondary antibody (1:4000) at room temperature for 2 hours. Protein bands were detected using enhanced chemiluminescence (ECL) reagents and measured with a Synoptics Syngene bioimaging system. Antibodies against PPARα, IL-1β, IL-18, β-actin (Abcam, Cambridge, MA, USA), NF-κB and caspase-1 (Novus Biologicals, CO, USA) were used.
2.7Immunohistochemical stainingThe liver tissue slices were subjected to repair antigen with high pressure cooking in citrate buffer for 15 minutes. Endogenous peroxidase activity was blocked with 3% H2O2, and 15 minutes later, the slices were incubated overnight at 4°C with primary antibodies (Abcam, Cambridge, MA, USA; Novus Biologicals, CO, USA; 1:100-200 dilution). The slices were washed three times with phosphate-buffered saline (PBS) and then incubated with the proper secondary antibody (ZSGB-BIO, Beijing, China) at 37°C for 30 minutes. Furthermore, the slices were washed three times with PBS, visualized with a 3,3’-diaminobenzidine (DAB) Detection Kit and counterstained with hematoxylin. Brown‒yellow staining represented a positive signal.
2.8Statistical analysisVariables were initially verified by normal distribution. All statistical results are presented as the mean±S.E.M. Comparisons of two groups were carried out with unpaired Student's t test, while comparisons of more than two groups were performed using one-way ANOVA with Tukey´s multiple comparison posttest. P<0.05 (two-tailed) was considered to indicate a significant difference.
2.9Ethical statementWritten informed consent was obtained from all individuals or their families included in the study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Medical Ethics Committee of the Third Affiliated Hospital, Hebei Medical University (W2021-087-1).
All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines.
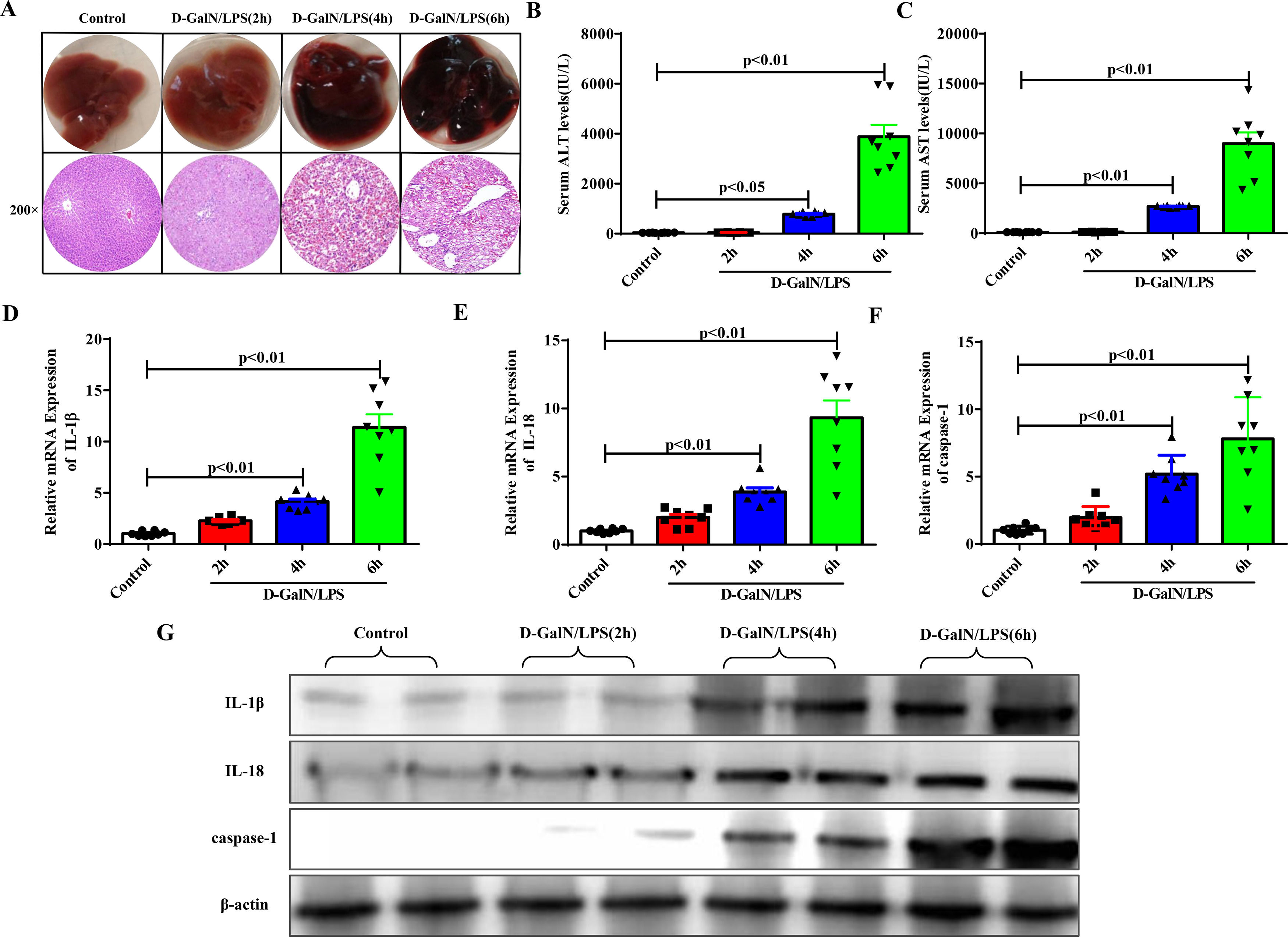
3Results3.1Proinflammatory cytokines and pyroptosis-associated protein expression increased with the gradual progression of ALFAccording to the time of euthanasia after D-GalN/LPS administration, we first divided the experimental mice into four groups: the normal control group and the ALF groups at 2 hours, 4 hours and 6 hours. D-GalN/LPS was injected intraperitoneally to induce ALF, and the same amount of normal saline was injected into the normal control group. Significant liver damage occurred 4 to 6 hours later, as determined by HE staining (Fig. 1A). Moreover, the elevated serum transaminase levels (ALT, AST) suggested similar results (Fig. 1B and Fig. 1C). The qRT-PCR (Fig. 1D to Fig. 1F) and western blotting results (Fig. 1G) demonstrated that with the progression of ALF, the expression of proinflammatory cytokines, such as IL-1β and IL-18, and the pyroptosis-associated protein caspase-1 was increased significantly.
Proinflammatory cytokines and pyroptosis-related protein had higher expression levels as ALF progressed. (A) The gross morphology and HE staining of livers in each group. (B-C) Serum transaminase levels in each group (n=8). (D-F) Gene expressions of IL-1β, IL-18 and caspase-1 were detected by qRT-PCR in each group (n=8). (G) Related proteins were detected by western blotting in the four groups of mice. Representative western blots of two samples from each group were displayed.
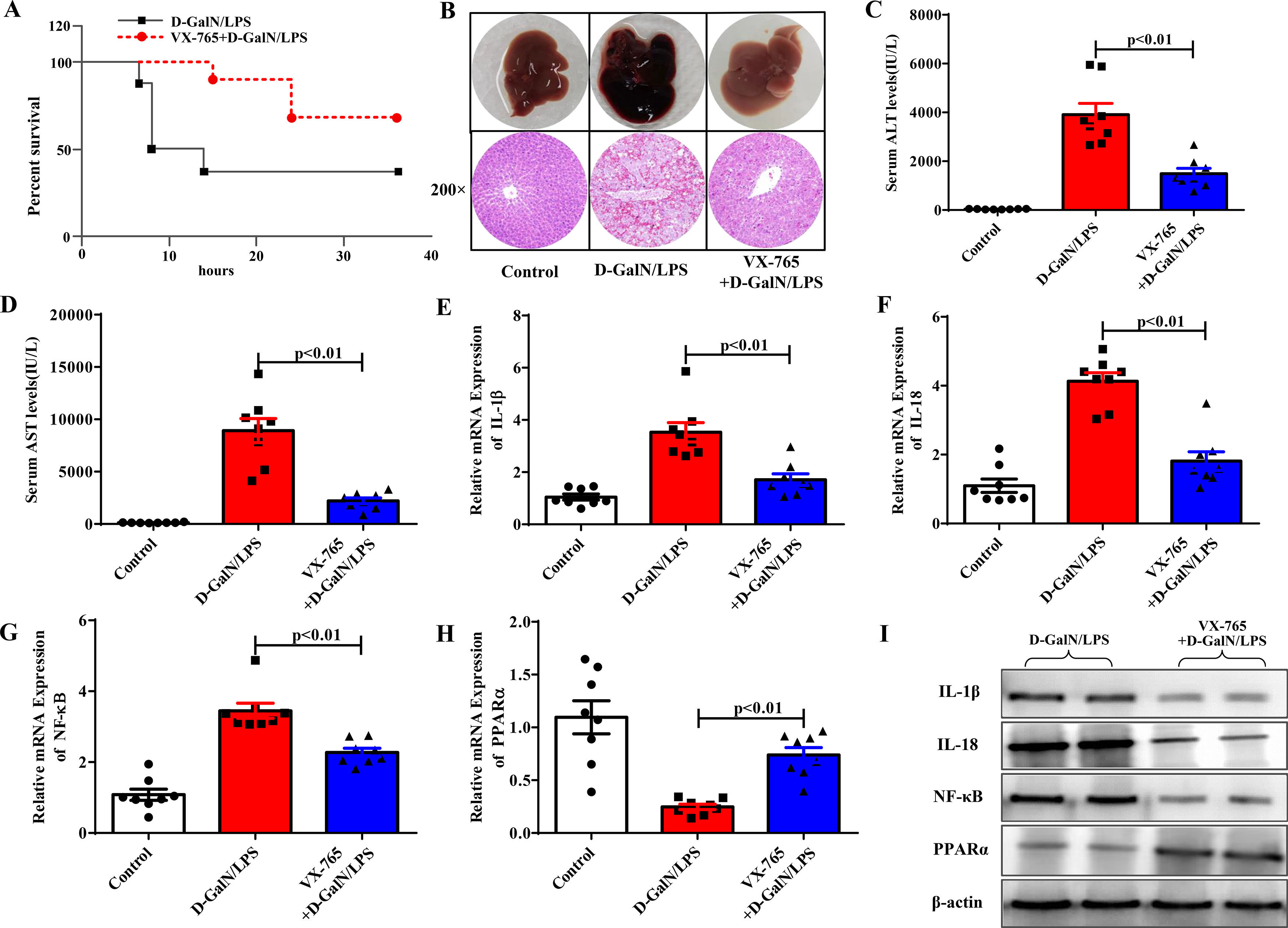
We then investigated the role of VX-765, a known pyroptosis inhibitor, in ALF. Intraperitoneal injection of VX-765 2 hours before D-GalN/LPS administration significantly reduced the mortality rate of the mice compared with the ALF group (Fig. 2A). Six hours after D-GalN/LPS injection, the mice were euthanized. Our results showed that VX-765 not only improved liver gross morphology and histopathological injury but also reduced serum enzyme levels (ALT, AST), proving that VX-765 could prevent ALF (Fig. 2B to Fig. 2D). Previous studies have shown that VX-765 can alleviate inflammatory responses by reducing the activity of the NF-κB pathway in other systemic diseases. We evaluated the effect of VX-765 on NF-κB in ALF. As shown in Fig. 2E to Fig. 2I, IL-1β, IL-18, and NF-κB mRNA and protein levels were significantly decreased, confirming the anti-inflammatory effect. Our previous study confirmed the role of PPARα in regulating inflammatory responses in ALF. Therefore, we examined the mRNA and protein levels of PPARα to determine whether it was a necessary mediator by which VX-765 could reduce inflammation. We observed that the gene and protein expression levels of PPARα were significantly upregulated compared with those in the D-GalN/LPS group (Fig. 2H and Fig. 2I).
VX-765 protected against ALF in vivo. (A) The survival rate of mice treated with D-GalN/LPS and VX-765+D-GalN/LPS was analyzed (n=10). (B) The gross morphology and HE staining of livers in each group. (C-D) Serum transaminase levels in the three groups (n=8). (E-H) Gene expressions of IL-1β, IL-18, NF-κB and PPARα were detected by qRT-PCR (n=8). (I) The associated protein expressions were analyzed by western blotting. Representative western blots of two samples from each group were displayed.
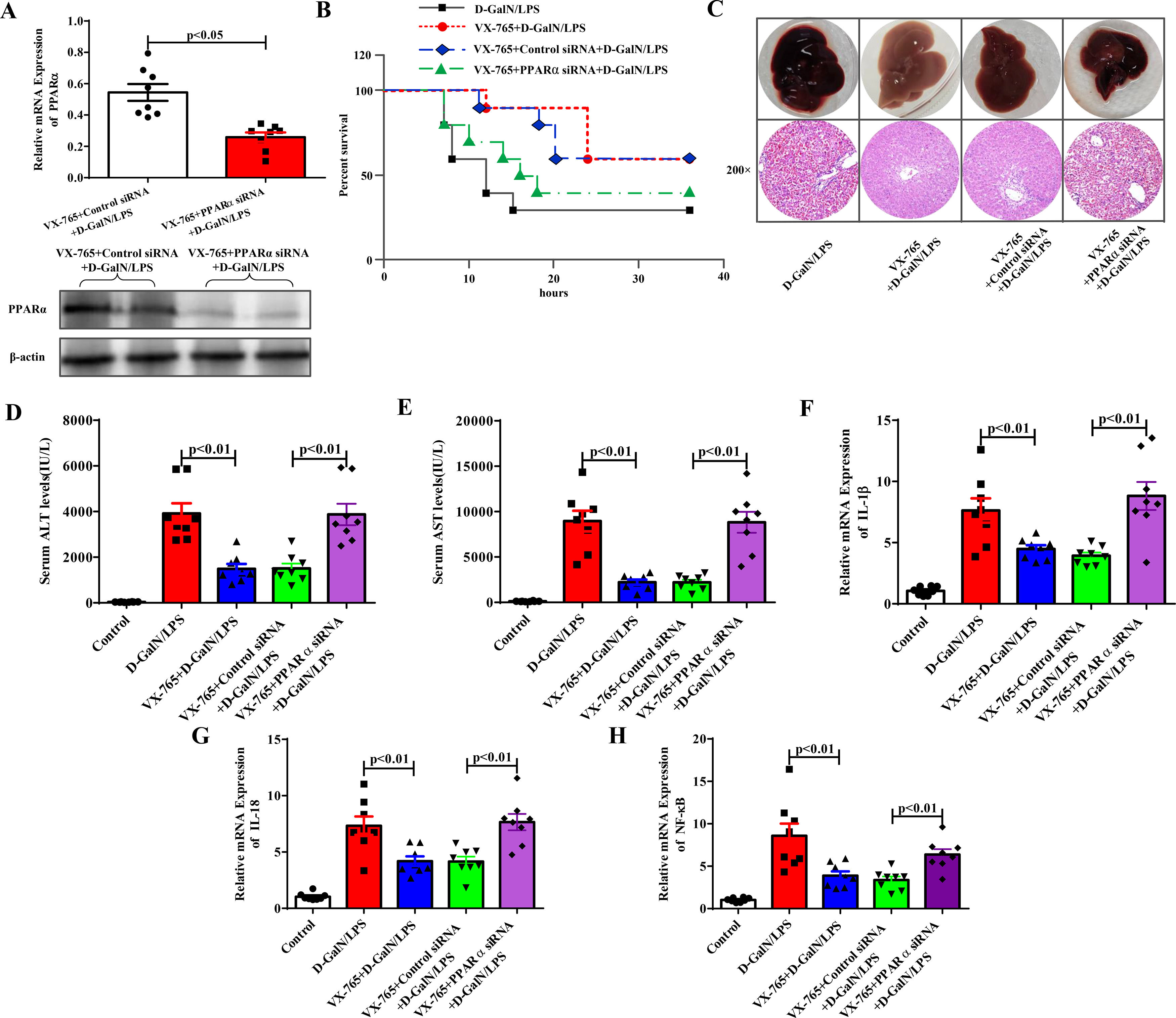
Next, we further revealed the protective mechanism by which VX-765 prevents ALF. PPARα protected against ALF in our previous study. By applying PPARα siRNA in the VX-765+D-GalN/LPS-treated group, PPARα inhibition was achieved. As shown in Fig. 3A, the expression level of PPARα was significantly reduced after PPARα siRNA was administered, as shown by the qRT‒PCR and western blotting results. The protective effect of VX-765 on the liver was significantly reduced, and there was a decrease in the survival rate (Fig. 3B), deterioration of overall liver morphology and pathological features (Fig. 3C), increased serum enzyme levels of ALT and AST (Fig. 3D and 3E), and increased IL-1β, IL-18 and NF-κB expression (Fig. 3F to Fig. 3H). These results suggested that in the presence of PPARα siRNA, the protective effect of VX-765 against ALF was reduced. Therefore, it could be concluded that VX-765 reduced inflammation by upregulating the expression level of PPARα, thereby protecting against acute liver failure.
Inhibition of PPARα attenuated the protective effect of VX-765 against ALF. (A) The gene and protein expression levels of PPARα from the VX-765+Control siRNA+D-GalN/LPS-treated mice and the VX-765+PPARα siRNA+D-GalN/LPS-treated mice were measured by qRT-PCR and western blotting respectively (n=8). (B) The survival rate of mice was analyzed (n=10). (C) The gross morphology and HE staining of livers from the D-GalN/LPS group, VX-765+D-GalN/LPS group, VX-765+Control siRNA+ D-GalN/LPS group and VX-765+PPARα siRNA+D-GalN/LPS group. (D-E) Serum transaminase levels from normal control and the above four groups (n=8). (F-H) Gene expressions of IL-1β, IL-18 and NF-κB were detected by qRT-PCR from each group (n=8).
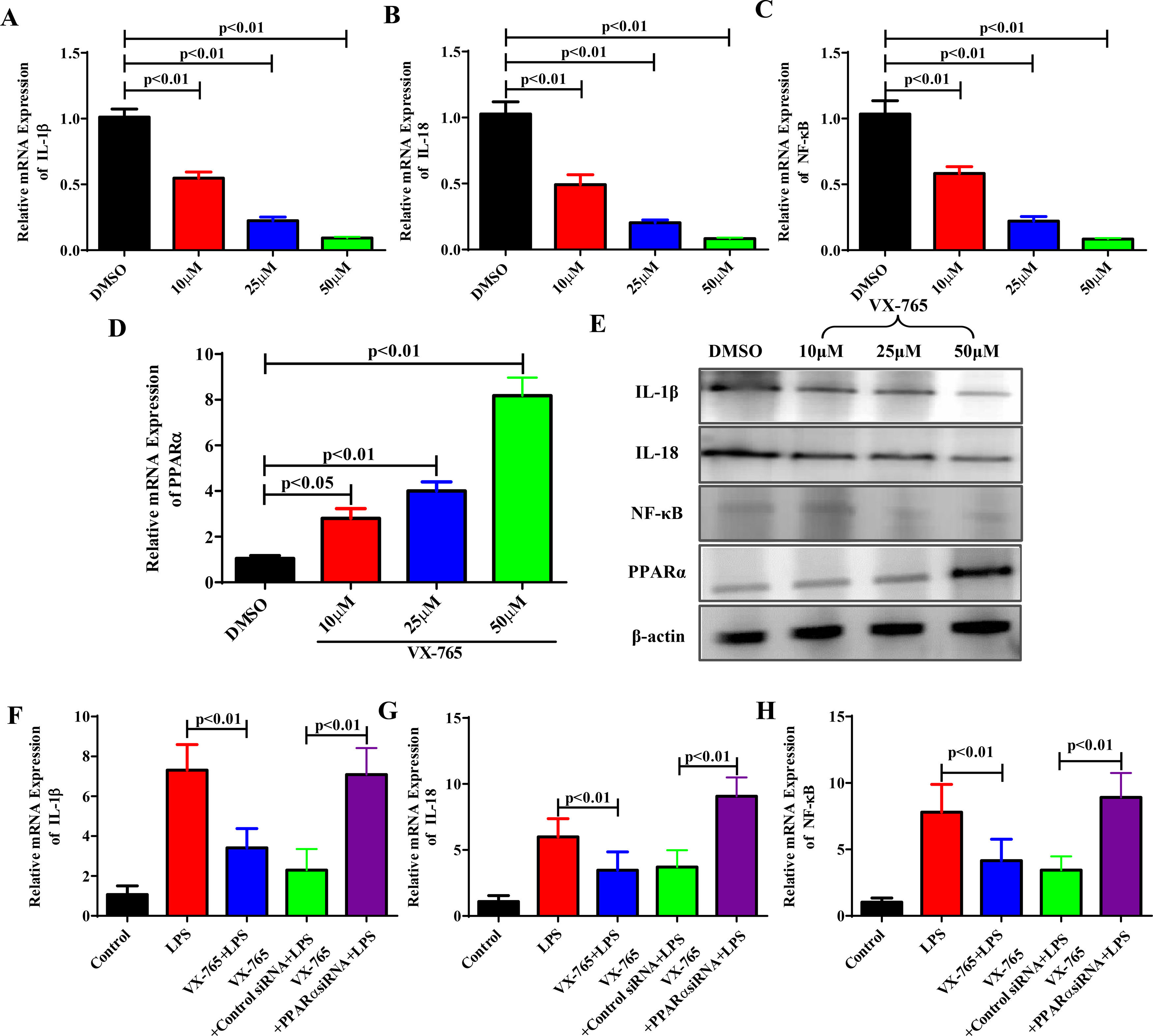
We know that VX-765 suppresses inflammatory responses by upregulating PPARα expression in vivo. To further confirm our findings in vivo, we studied the effects of VX-765 on the inflammatory responses and cellular mechanism. LO2 cells were treated with VX-765 (10, 25 and 50 μM) for 2 hours and then with LPS for 6 hours. With increasing VX-765 concentrations, IL-1β, IL-18 and NF-κB expression levels decreased, while PPARα expression rose gradually in a dose-dependent manner (Fig. 4A to Fig. 4E). To further confirm the cellular mechanism, we examined whether VX-765 reduced inflammatory responses by upregulating PPARα in vitro. As shown in Fig. 4F to Fig. 4H, when PPARα expression was inhibited by siRNA, the anti-inflammatory effect of VX-765 on LPS-stimulated LO2 cells was significantly reduced.
VX-765 inhibited inflammatory responses by upregulating PPARα expression in vitro. (A-D) After being treated with VX-765 at concentrations of 10μM, 25μM and 50μM respectively for 2 hours, LO2 cells were then treated with LPS for 6 hours with the concentration at 20 ng/ml. The gene expressions of IL-1β, IL-18, NF-κB and PPARα were detected by qRT-PCR at different concentrations of VX-765. (E) Protein expressions of IL-1β, IL-18, NF-κB and PPARα were analyzed by western blotting for groups with different concentrations of VX-765. (F-H) Gene expressions of IL-1β, IL-18 and NF-κB were detected by qRT-PCR for the normal control, LPS, VX-765+LPS, VX-765+Control siRNA+LPS and VX-765+PPARα siRNA+LPS groups respectively.
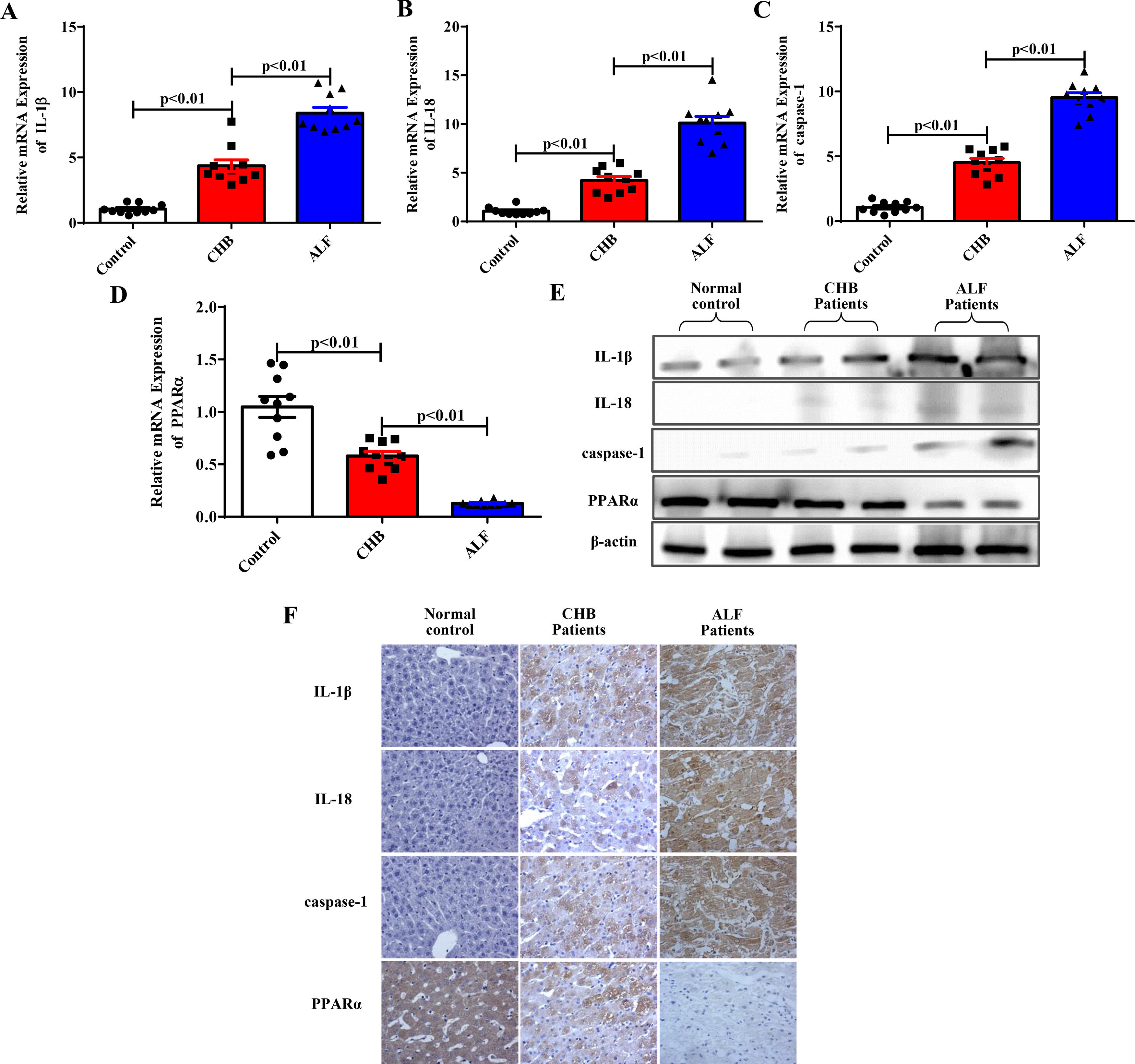
We then confirmed whether inflammation, pyroptosis, and PPARα were related to the progression of ALF in patients. The expression levels of IL-1β, IL-18, the pyroptosis-associated protein caspase-1 and PPARα in 10 ALF patients infected with HBV, 10 CHB patients and 10 healthy controls were determined. As shown in Fig. 5A to Fig. 5D, the mRNA levels of IL-1β, IL-18 and caspase-1 were significantly increased, while PPARα expression decreased with the progression of ALF according to the qRT‒PCR results. Furthermore, western blot and immunohistochemical experiments verified these results in liver tissues (Fig. 5E to Fig. 5F). Our experiments showed that proinflammatory cytokines and pyroptosis-related protein expression was increased, while PPARα expression was downregulated with the progression of ALF in clinical patients.
The related gene and protein expression levels in individuals with ALF. (A-E) The expression levels of IL-1β, IL-18, caspase-1 and PPARα were analyzed by qRT-PCR (n=10) and western blotting methods for the three groups. Representative western blots of two samples from each group were displayed. (F) Staining of IL-1β, IL-18, caspase-1 and PPARα in the three groups by immunohistochemistry.
As a newly developed pyroptosis inhibitor, VX-765 is an oral medicine in development that is designed to inhibit the activation and expression of caspase-1 [21–24]. Previous studies have revealed that VX-765 can reduce the expression level and activity of caspase-1 in the heart, prevent myocardial infarction, downregulate the levels of inflammatory cytokines in patients with diabetic nephropathy and attenuate the functional outcomes of mice with traumatic brain injury [25–28]. However, the effect and mechanism of VX-765 on ALF remain unclear. The main novel finding of this study is that VX-765 upregulates PPARα expression to reduce inflammatory responses, thereby protecting against acute liver failure.
A large number of studies have previously been carried out on the pathophysiology of ALF; however, the mechanism by which organ damage occurs has not been fully revealed. ALF remains a rare but deadly disease if poorly treated [29,30]. We need to spare no effort in improving liver support in clinical practice, which may give ALF patients the time necessary for spontaneous recovery or until liver transplantation is available. Exploring new effective treatments for ALF patients is of vital importance, and more studies are needed for this patient population [31]. Understanding disease progression and multiple organ failure development is necessary for further improvements in survival.
ALF features a sudden loss of liver function due to overwhelming hepatocyte death and liver inflammation [32,33]. Liver inflammation plays a substantial role in ALF pathogenesis [34,35]. The mouse model of peritoneal injection of D-GalN plus LPS is a widely used animal model to study ALF. It is an ideal animal model that has good repeatability, and liver injury is akin to clinical ALF, while extrahepatic toxicity is not obvious [36–38]. Therefore, in this study, we used D-GalN/LPS-treated mouse models to further examine the pathogenesis of ALF.
As a new mode of programmed cell death, pyroptosis is closely related to inflammatory reactions. During this process, caspase-1 is activated, and large amounts of proinflammatory cytokines such as IL-1β and IL-18 are released [39,40]. Previous experiments have shown that inflammatory responses can be reduced by inhibiting pyroptosis, and the NF-κB signaling pathway is closely associated with this process [41,42]. In our experiments, proinflammatory factors and pyroptosis-related protein expression increased with the progression of ALF, and for the first time, we revealed that inhibiting pyroptosis with VX-765 significantly relieved liver damage and protected against ALF. To explore the underlying protective mechanism of VX-765 in ALF, we examined the effect of VX-765 on inflammation and found that VX-765 could inhibit inflammation and regulate the activity of NF-κB in vitro and in vivo.
PPARα, which is a nuclear transcription factor, is widely involved in inflammatory responses, apoptosis and lipid metabolism [43–45]. Our earlier studies showed that PPARα could protect against acute liver failure, and numerous experiments have demonstrated that PPARα acts as a mediator in a variety of diseases [46–48]. Furthermore, we hypothesize that PPARα may regulate VX-765-mediated inhibition of inflammation during ALF progression.
In the present study, we examined PPARα and inflammatory cytokine expression levels in D-GalN/LPS-treated mice, in LPS-stimulated LO2 cells and in clinical ALF patients. We found that VX-765 could upregulate PPARα expression in vivo and in vitro. Moreover, PPARα expression gradually increased while inflammatory cytokine expression decreased with increasing concentrations of VX-765 in a dose-dependent manner. Furthermore, inflammation was aggravated when PPARα siRNA was used to inhibit PPARα expression, which was accompanied by the exacerbation of liver injuries. These results suggested that the protective effect of VX-765 against ALF was attenuated when PPARα siRNA was applied, which meant that VX-765 could reduce inflammatory responses by upregulating PPARα expression to protect against acute liver failure.
5ConclusionsThis study deepened our understanding of the ALF mechanism and provided a fresh perspective on the significance of VX-765 in reducing liver inflammatory responses and protecting against ALF by upregulating PPARα expression. Therefore, VX-765 could improve the outcome of ALF, and oral administration might become a clinical treatment strategy for ALF patients [22,23].
FundingThis work was supported by Hebei Medical Science Research Project Plan (20221153).
Authors' contributionsMingjing Jiao and Caiyan Zhao devised the experiments. Mingjing Jiao and Jiachao Wang conducted the experiments and drafted the manuscript. Hongzhu Yin was in charge of the pathological observation. Xin Zhao and Wenpeng Liu collected the samples. Yanjun Qin and Chunhuan Zhang performed statistical analysis. The final submitted manuscript has been read and approved by all authors.