Introduction
Type 2 diabetes mellitus is a chronic disease with significant morbility and mortality, and a growing incidence in developed countries. All the patients require medical advice and the majority need medication. Although many therapeutic agents exist, it is not clear which of them, and which patient sub-groups, produces more benefit in primary results, such as mortality, coronary disease, stroke, nephrotic disease, arterial disease retinopathy.1
Metformin is a biguanide which increases the peripheral and hepatic sensitivity to insulin, improves the fasting and post-prandial glycemic profile,2 and lacks significant risk in producing lactic acidosis, which has been reported previously with another biguanide.3
There are 2 published meta-analyses of metformin compared to sulphonylureas4,5 and to diet,5 which present secondary results (changes in weight, glycemia, and lipids), but do not report primary results.6
This review has as its aim, to answer 2 questions: if the use of metformin in monotherapy, compared with any other treatment, in type 2 diabetics is associated with beneficial changes in primary and secondary results and if there is any sub-group of patients with type 2 diabetes who might benefit more from treatment with metformin.
Patients and Methods
The inclusion criteria have been: randomized clinical trials, open, simple or double blind, published or unpublished, in any language, on metformin in monotherapy (with metformin as primary treatment option, or changing to it from a different treatment used previously), compared with placebo, diet, *-glucosidase inhibitors, meglitinides, sulphonylureas, thiazolidinediones, or insulin, in the type 2 diabetic population, recruited in general medical or diabetic clinics, with pharmacological treatment for a minimum of 3 months, and which reported primary or secondary results.
As primary results any event associated with diabetes has been searched for (death, myocardial infarction, angina, stroke, nephrotic disease, peripheral arterial disease, vitreous hemorrhage, retinopathy, and cataracts), mortality associated with diabetes, total mortality and microvascular disease. Secondary results included: glycosylated hemoglobin A1 (HbA1c), fasting glucose, weight or body mass index (BMI), lipids, C peptide, insulinemia, systolic and diastolic blood pressure, microalbuminemia, and adverse reactions.
A search has been carried out on MEDLINE (1966 to 2003), EMBASE (1974 to 2003), LILACS (1986 to 2003), and the Cochrane Library (part 3, 2003), using the terms: "metformin," "biguanide," "type 2 diabetes," "non-insulin dependent diabetes," "random," and "clinical trial." The lists of references of the relevant studies obtained have been analyzed. Two reviewers (A.S.C. and I.F.E.), independently, evaluated each title and summary. If the reference appeared to comply with the inclusion criteria, a complete copy of the article was obtained. Contact was made with the manufacturers of the medicine to obtain additional references (although no new reference was added), and with some authors to resolve doubts. Duplications were eliminated. Two reviewers (A.S.C. and I.F.E.), independently, extracted the data and scored the quality.7,8 The studies were divided into 2 quality groups: high and medium-low. The quality was considered high when the study obtained 4 or 5 points on the Jadad scale7 and it has mentioned that reasonable allocation concealment had been attempted.8 To incorporate the evaluations of the validity of the studies a sensitivity analysis was used, using the inclusion and exclusion of the studies of medium-low quality, and a meta-regression including the quality as a co-variable.1 The origin and authorship of the studies were not concealed from the reviewers. Discrepancies were resolved by consensus. The inter-observer agreement was analyzed using the kappa9 statistic, which was 0.85, which indicated substantial agreement.
For the calculations RevMan version 4.2 and Stata version 5 were used. The weighted mean differences (WMD) have been calculated for data on the same scale and the standardized mean differences (SMD) for different scales. Whenever possible the mean of the change ± its standard deviation was used,10-16 and the final results for the rest,17-37 carrying out an intention to treat analysis. The dichotomic data were included as number of events and relative risk (RR). We have summarized the data in a total combined result using the random effects model due to the heterogeneity detected. We carried out an analysis of sensitivity based on: double blind trials, high quality trials, and trials with concealed allocation. To calculate the presence of bias of publication we used the Begg38 correlation test and the Egger graph.39 We have evaluated heterogeneity using the Z marker, the *2 statistic and the I2 statistic (values of I2 greater than 50% were considered excessive),40 with meta-regression.
Analysis of subgroups was carried out to examine the influence of cardiovascular risk factors on the size of the effect and also if any sub-group could benefit from treatment with metformin to a greater extent. These sub-groups were: obesity (BMI>30 kg/m2 or weight greater than 85 kg), hypertension, low density lipoprotein cholesterol (LDL-C>3.99 mmol/L), fibrinogenemia, platelet function, and over 65 years.
Results
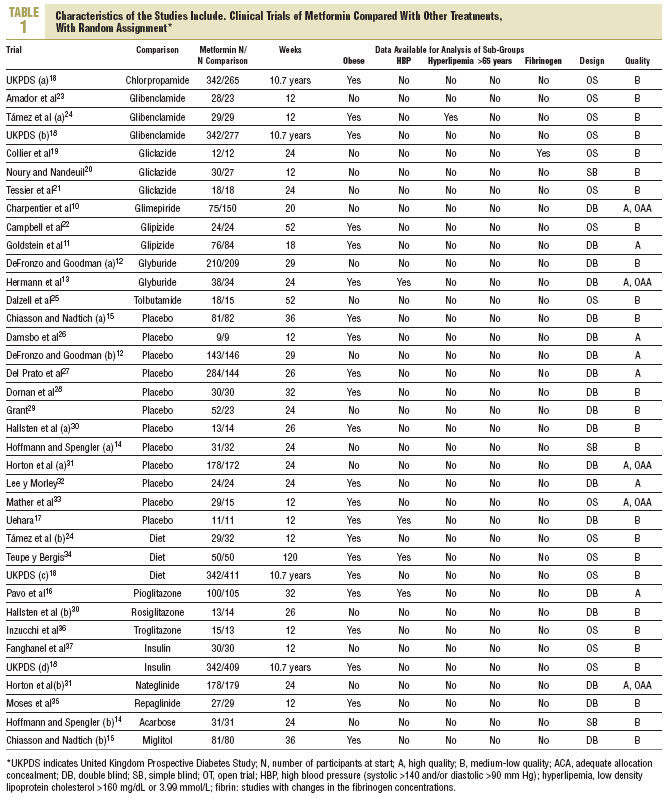
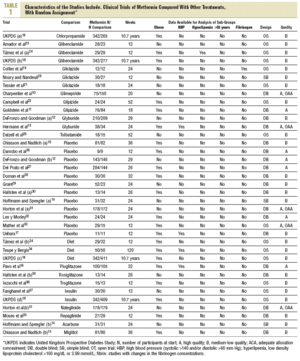
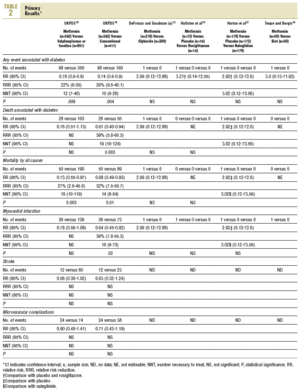
In the search 1306 different references were identified and 215 considered relevant. 179 were excluded for not complying with the inclusion criteria and 7 crossed studies for not offering data at the first cross. They included 29 randomized clinical trials10-37 (with 37 treatment groups and 5259 participants) which compared metformin (29 trials and 2007 participants) with sulphonylureas (13 studies and 1167 participants),10-13,18-25 placebo (12 studies and 702 participants,12,14,15,17,26-33 diet (3 studies and 493 participants),18,24,34 thiazolidinediones (3 trials and 132 participants),16,30,36 insulin (2 trials and 439 participants),31,35 meglitinides (2 studies and 208 participants),31,35 and *-glucosidase inhibitors (2 trials and 111 participants).14,15 Metformin was used in doses up to 3 g (range, 1-3 g). A clinical trial was obtained before publication.17 The mean age of the patients was 56 years (range, 35-73), the majority were white race and 50% were women. The mean duration of the trials was 5 months (range, 3-24), and 10.7 years for the UK Prospective Diabetes Study (UKPDS).18 Ten studies were considered high quality. Four had adequate concealed allocation (Table 1).
The Begg and Egger tests indicated that there had been no publication biases.38,39 With the exception of obesity, which was recorded in 17 studies, data in the anticipated subgroups could not be combined. Only 4 studies included hypertensive patients and 2 hyperlipemic ones. One trial studied platelet function19 and none offered data specific to over 65 years.
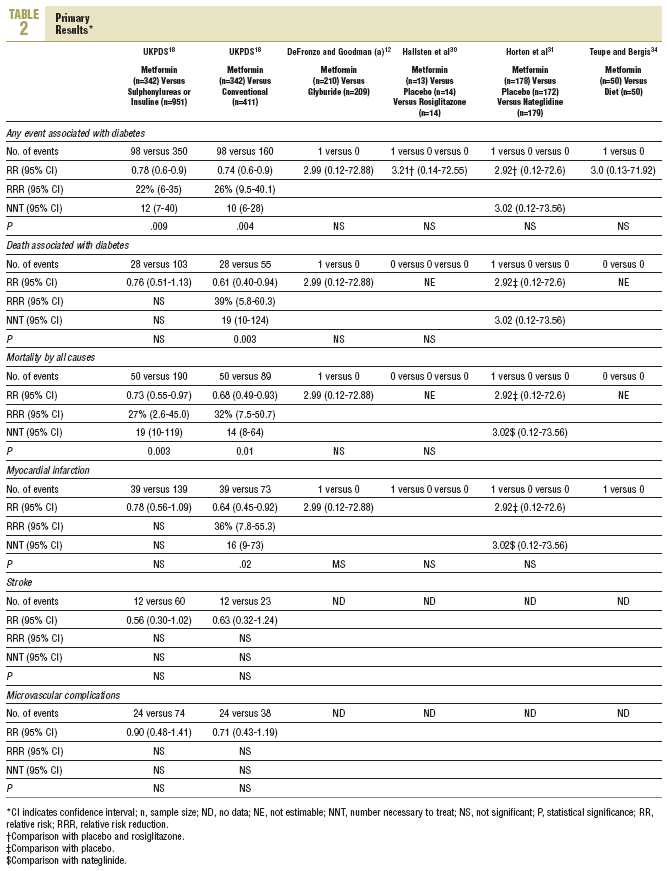
Primary Results (Table 2)
Five studies reported primary results12,18,30,31,34 and another 4 specifically reported they did not have them.14,16,35,36 In the UKPDS, in overweight patients,18 metformin in monotherapy (n=342) showed a greater benefit than chlorpropamide, glibenclamide, or insulin (n=951); in all of them intensive glycemic control was attempted (fasting glucose <106 mg/dL) for any event associated with diabetes (98 vs 350; RR=0.78; 95% confidence interval (CI), 0.65-0.94), and for total mortality (50 vs 190; RR=0.73; 95% CI, 0.55-0.97). Metformin was compared with non-intensive conventional treatment (fasting glucose <270 mg/dL; n=411 patients) and showed a greater benefit for any event associated with diabetes (98 vs 190; RR=0.74; 95% CI, 0.60-0.90), death associated with diabetes (28 vs 55; RR=0.61; 95% CI, 0.40-0.94), total mortality (50 vs 89; RR=0.68; 95% CI, 0.49-0.93) and myocardial infarction (39 vs 73; RR=0.64, 95% CI, 0.45-0.92). In the other 4 clinical trials they reported 4 myocardial infarctions, 2 of them fatal, and all the events were registered in the patients in the metformin group (4 vs 0; RR=3.58; 95% CI, 0.73-17.52).
Primary results were not reported in clinical trials with hyperlipemic patients, nor were any data offered from patients over 65 years old. A small trial recorded, non significant changes in fibrinogen.
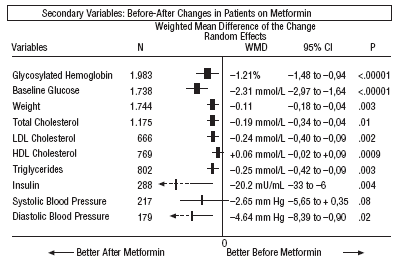
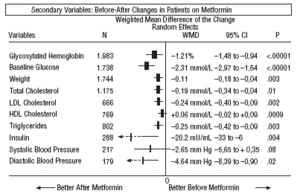
As regards the secondary results (Figures 1-4), in the comparison before and after metformin (Figure 1) the patients on metformin showed a significant reduction in HbA1c (WMD=1.21%; 95% CI, 1.48 to 0.94), glycemia (WMD=2.31 mmol/L; 95% CI, 2.97 to 1.64), cholesterol (WMD=0.19 mmol/L; 95% CI, 0.34 to 0.04), LDL cholesterol (WMD=0.24 mmol/L; 95% CI, 0.40 to 0.09), triglycerides (WMD=0.25 mmol/L; 95% CI, 0.42 to 0.09), insulin (WMD=20 mU/ml; 95% CI, 33 to 6), diastolic pressure (WMD=4.64 mm Hg; 95% CI, 8.39 to 0.90), and weight (SMD=0.11 kg; 95% CI, 0.18 to 0.04).
Fig. 1. Beforeafter changes in patients on treatment with metformin.
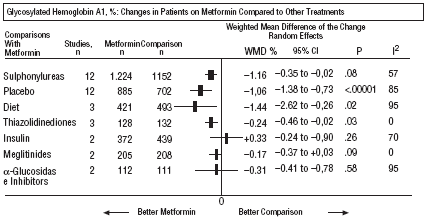
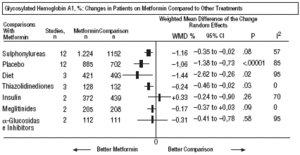
Fig. 2. Combined glycosylated hemoglobin A1.
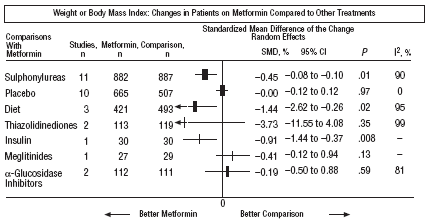
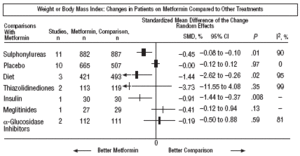
Fig. 3. Combined weight results.
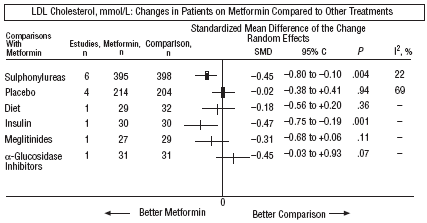
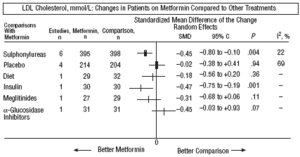
Fig. 4. Combined low density lipoprotein cholesterol results (LDL).
In comparison with patients on treatment with sulphonylureas, patients on metformin showed a greater benefit for glycemia (WMD=0.31mmol/L; 95% CI, 0.57 to 0.05), LDL cholesterol (WMD=0.22 mmol/L; 95% CI, 0.35 to 0.10), triglycerides (WMD=0.24 mmol/l; 95% CI, 0.46 to 0.02), and weight (BMI) (SMD=0.45 kg/M2; 95% CI, 0.80 to 0.10). A small clinical trial 23 showed a benefit of metformin for microalbuminuria (WMD=53 mg/day; 95% CI, 86 to 19). The meta-regression analysis did not show any significant explained variable of heterogeneity. These results were consistent with the sensitivity analysis of double blind trials. In the sub-group of overweight patients, metformin had a greater benefit on weight (BMI) (SMD=0.58 kg/m2; 95% CI, 1.00 to 0.16), cholesterol (WMD=0.38 mmol/L; 95% CI, 0.55 to 0.20) and LDL cholesterol (WMD=0.27 mmol/L; 95% CI, 0.40 to 0.14). There were more cases of hypoglycemia in the patients who received sulphonylureas (P=.004) and more diarrhea with metformin (P=.02).
In comparison with the placebo, metformin showed a greater benefit for HbA1c (WMD=1.06%; 95% CI, 1.38 to 0.73) and glycemia (WMD=1.84 mmol/L; 95% CI, 2.38 to 1.30). The meta-regression indicated metformin had a greater effect on obesity (P=.001) and in studies where a diet was not enforced (P=.001). The results were consistent with the sensitivity analysis in double blind trials on obesity, of high quality and with adequate concealed assignation. There were more cases of diarrhea with metformin (P=.005).
In comparison with diet, metformin showed greater benefit for HbA1c (WMD=1.44%; 95% CI, 2.62 to 0.26), cholesterol (WMD=0.61 mmol/L; 95% CI, 0.93 to 0.29), insulin (WMD=60 mU/mL; 95% CI, 79 to 40), and C peptide (WMD=0.61 nmol/L; 95% CI, 0.89 to 0.33). There was more hypoglycemia with diet (P=.01).
Compared with thiazolidinediones, showed a greater benefit for HbA1c (WMD=0.24%; 95% CI, 0.46 to 0.02).
Metformin improved weight compared to insulin (SMD=0.91 kg; 95% CI, 1.44 to 0.37), cholesterol (WMD=0.42 mmol/L; 95% CI, 0.69 to 0.15), LDL cholesterol (WMD=0.47 mmol/L; 95% CI, 0.75 to 0.19), the systolic pressure (WMD=9.50 mm Hg; 95% CI, 15.15 to 3.85), and the diastolic (WMD=7.00 mm Hg; 95% CI, 9.41 to 4.59).
Compared with the meglitinides, metformin showed greater benefit for glycemia (WMD=0.73 mmol/L; 95% CI, 1.18 to 0.28). There were no differences in the rest of the comparisons. There were more cases of diarrhea in the metformin group (P=.0002).
On comparing metformin with *-glucosidase inhibitors, the cholesterol levels improved in the patients on acarbose (WMD=1.92 mmol/L; 95% CI, 1.20-2.64). There were more digestive problems in the metformin group (P=.002).
There were no cases of lactic acidosis in the 29 clinical trials.
Discussion
The UKPDS18 prospective study continues to be the longest study to date (median of 10.7 years and 4075 participants). In overweight patients, attempting intensive glycemic control, metformin in monotherapy was beneficial compared with intensive control with chlorpropamide, glibenclamide or insulin, thus reducing the incidence of any event associated with diabetes and total mortality. It was the same when compared with a conventional treatment (mainly diet), since it reduced the incidence of any event associated with diabetes, mortality and myocardial infarction. These results confirm the findings of the UKPDS, although we have not found the benefit reported for stroke (RR=0.56; 95% CI, 0.30-1.02), probably because we did not have access to individual data.18
The study of the effect of the addition of metformin to other medications has not been the objective of this review, but it has to be mentioned that in the UKPDS the benefit in the primary results were not able to demonstrate this on adding it to a sulphonylurea. It reported an increase in mortality in the metformin-sulphonylurea treatment without overweight group compared to sulphonylurea only, although as an explanation baseline differences between patients were adjusted.18 Despite the doubts created, the recent clinical trials of metformin combined with other treatments have yet to report primary results, in combination with sulphonylureas41 as well as with insulin.42
No clinical study lasting longer than 8 months has been found which compares new oral agents (new sulphonylureas, *-glucosidase inhibitors, meglitinides, or thiazolidinediones) with metformin to be able to make a comparison of primary results, which it makes it difficult to recommend new drugs instead of metformin as the first therapeutic option, since the moderate benefits in isolated secondary results have still to show benefit in primary results. In fact, the UKPDS has demonstrated that there is a benefit in morbimortality with intensive glycemic control, but given similar control in all groups, the possible benefit of metformin could not be due only to its glycemic control and
other possible effects on platelet aggregation and thrombolysis have been put forward as a hypothesis.43
It must not be forgotten that diabetes added to other cardiovascular risk factors increases the risk of coronary disease and stroke, and that in a sub-study of the UKPDS the cardiovascular benefit from controlling hypertension was better than that of hyperglycemia.44 However, with the exception of a greater benefit with metformin in overweight patients, this review has not found and has not been able to combine results specific to diabetic sub-groups such as in hypertensives, hyperlipemics, with fibrinolysis disturbances or over 65 years. For this reason it would be advisable that future investigators make an effort in recruiting these populations to better apply their results to our usual patients.
In the combined result of the 29 studies, patients on metformin had considerably improved glycemic control (HbA1C by 1.21% and glucose by 2.31 mmol/L) and a modest decrease in weight, lipid values, and diastolic pressure. Patients on metformin also achieved a greater benefit in glycemic control than those assigned to a placebo, diet or thiazolidinediones, and a greater benefit in weight control and LDL cholesterol than those assigned to sulphonylureas or insulin. On the other hand, the more modern agents such as the thiazolidinediones and meglitinides did not provide any greater benefit in glycemia, lipid values, weight, or blood pressure than metformin.
In this review, which includes 2007 patients assigned to treatment with metformin a minor reversible adverse effect was found (diarrhea), but no cases of lactic acidosis, which confirms the safety evidence of metformin, which has been published in a systematic review.3
One limitation of this review is the low number of existing double blind clinical trials, lack of data on the allocation concealment and the heterogeneity. The latter has been studied by meta-regression and 2 variables have been found which can explain it. One is the greater benefit of metformin in the overweight population and the other the attempt to enforce the diet by protocol, which could be an independent factor in the improvement of glycemia. It is probable that in establishing these protocols in this type of clinical study biases in fulfillment are avoided.
There were also some noteworthy pints: the combined effects were consistent in the sensitivity analysis, no publication biases were found and there was no significant disagreement between trials, despite their differences in design and quality. The secondary results are concordant with the 2 systematic reviews carried out previously, although the contribution of this review is the widening of the study to all anti-diabetic medications used in this disease, including insulin and diet, as well as recording the primary results, which are of most interest to the patients.4,5
To sum up, in the long term metformin, in single intensive treatment in overweight patients with type 2 diabetes, compared with intensive treatment with glibenclamide, chlorpropamide, or insulin and with conventional treatment shows greater benefits in primary results. No other anti-diabetic has been analyzed in comparison with metformin, thus this review supports the usefulness of this as a primary therapeutic option in type 2 diabetes mellitus, where it plays an important role in the prevention of vascular complications.
Acknowledgements
To Aureli Tobías, for help in the statistical methods of publication biases, and to Dr Miguel Brito, for his critical reading of the manuscript.