Introduction
The publication of suspected adverse drug reactions (ADRs) in medical journals continues to have a prominent role as a method of pharmacovigilance (PV), despite the criticisms produced over the low level of evidence in the communication of isolated cases and due to the delay between the detection of the ADR and its publication.1 PV is considered to have begun in 1961, when McBride published several cases of phocomelia due to thalidomide in the letters to the editor section (LE) of The Lancet. Recently, Arnaiz et al, in 2001, demonstrated that the publication of isolated cases continues to be of great importance, by confirming that of the 22 drugs withdrawn from the Spanish market for safety reasons in the 1990´s, the decision was based on the publication of cases in medical journals in 59% of them.2
However, to prevent causing false alarms the communication of cases of ADRs need to comply with some minimum criteria which can guarantee their quality.3-5 In 1982, Venulet showed that the information considered minimal featured in only 21% of the publications of ADRs,6 a situation which has since improved substantially from the beginning of the 1990's.7
In Spain, studies carried out on the publication of cases of ADRs have been limited and have focussed mainly on aspects such as causal relationship or the inclusion of a group of minimum criteria.8,9 However, there are no studies which analyse other aspects of ADR publications, such as patient characteristics or the importance of the publication.
The objectives of the present study have been to assess the characteristics, the causal relationship, quality of documentation (or the appearance of a group of minimum criteria) and relevance of the publication of suspected ADRs in the Letters to the Editor section of four Spanish internal and general medicine journals.
Pacients and methods
Design
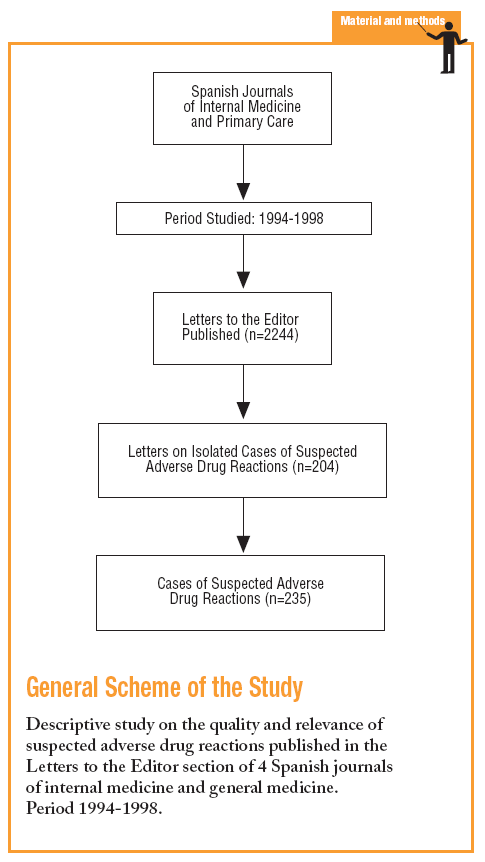
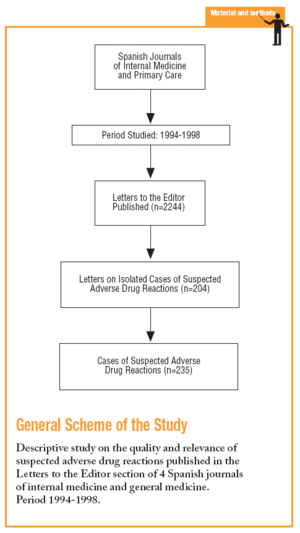
It is a descriptive study of isolated cases of ADRs published in the LE section of four internal medicine (IM) or general medicine (GM) Spanish journals between 1994 and 1998.
Study Population
The first four journals from the "Citation Index and Bibliometric Indicators of Journals of Internal Medicine and its Specialties 1990-1991", according to the number of citations: Medicina Clínica, Revista Clínica Española, Atención Primaria, and Anales de Medicina Interna.10 A researcher reviewed, selected and documented the LE which were reporting ADRs, and a group of doctors with experience in PV evaluated them and reached a consensus.
Measurements
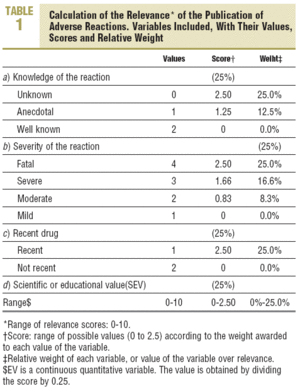
Variables studied. a) From the journals: LE published; b) origin and notification of the ADR: year of publication, health care source (primary care, hospital, pharmacy service, clinical pharmacology service, PV service, emergency service, or other), and notification to the PV service; c) on the patients: age, sex, weight, and disease history; d) on the drugs administered: total and type of drugs (recent or non-recent), active ingredient, dose, indication administration route, and period of treatment; e) the adverse reaction and its latency period; f) the causality algorithm; g) the severity of the reaction; h) the quality of the documentation; i) the scientific or educational value, and j) the relevance of the publication.
The definition of ADR is that used by the Spanish PV System.11 The causal relationship was established using the Spanish PV System algorithm, which classifies 5 categories of causality
(attributability): improbable (¾0 points), conditional (1-3 points), possible (4-5 points), probable (6-7 points) and definitive (>=8 points).12
The coding of the drugs was carried out using the European Pharmaceutical Market Research Association Anatomical Classification of Pharmaceutical Products. The reasons for prescribing were coded using International Classification of Diseases. It was considered a recent drug if its marketing in Spain had been carried out in the 5 years preceding the publication. The WHO terminology was used for coding the ADR.
The severity was determined by applying the scale used by the Spanish PV System: mild, moderate, severe, lethal, or unable to code.13
The quality of the documentation was defined by the appearance of a series of criteria considered as minimum3-5:
Associated with the patient:
1. Age.
2. Gender.
3. Weight.
4. Disease history.
Associated with the drug involved:
5. Indication.
6. Dose.
7. Administration route.
8. Period of administration.
Others:
9. Dosing regimen of the drugs not implicated (indication, dose, route, and period of administering).
Associated with the ADR:
10. Latency period.
11. Start and end.
12. Performing pertinent complementary examinations.
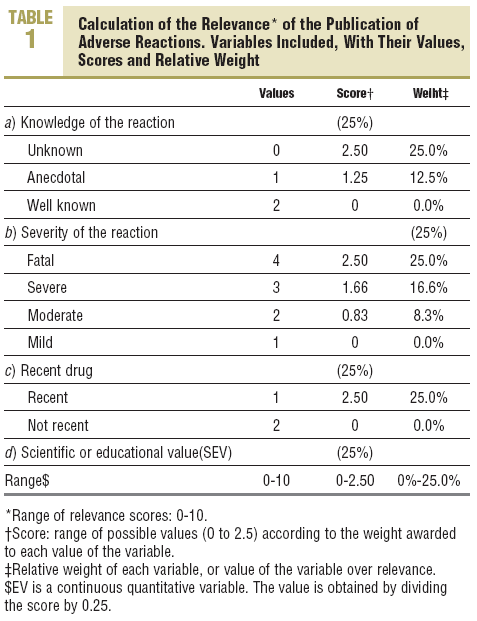
Each criterion is awarded one point; thus, the quality of documentation varied between 0 and 12, the scientific or educational value was obtained by calculating the mean of the subjective scores of the members of the evaluation group, and its range varied between 1 and 10, with a maximum of 5 for scientific value and another 5 for educational value. For the scientific value, consideration was given if the publication contributed any new aspect on the ADR, if it was well documented, or if it postulated any new mechanism of a previously known ADR. For educational value, consideration was given if the publication could lead to changes in prescribing the drug involved.
The relevance of the publication of ADRs was defined, from a proposal by Meyboom et al,14 by 4 variables: severity, previous knowledge, scientific or educational value, and type of drug involved (recent or not). Its value fluctuated between 0 and 10 (Table 1).
Statistical Analysis
The results are presented as percentages for the qualitative variables and as arithmetic mean ± standard deviation (SD) for the quantitative ones. A bivariate comparison was performed.
A 95% was taken as the limit of statistical significance. The results are shown in the Tables and Figures. The software programs SPSS 1996 and Office 97 were used.
Results
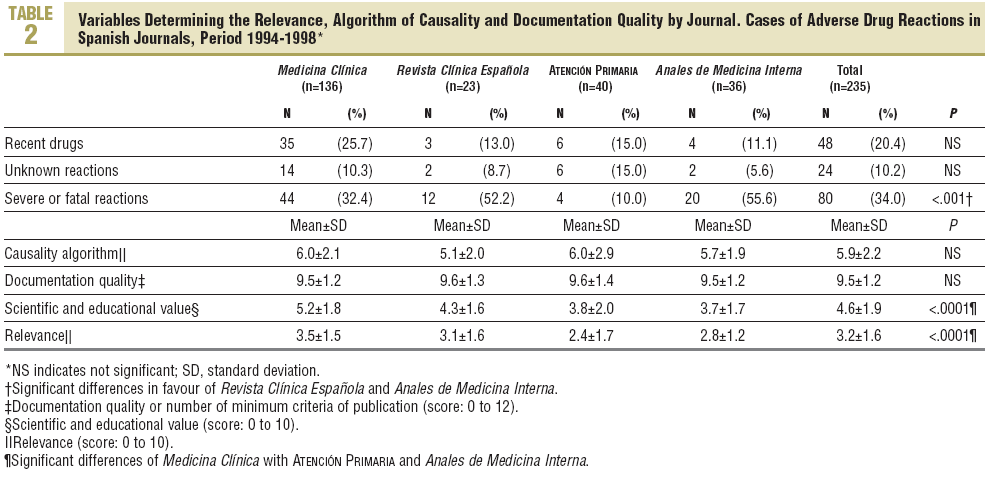
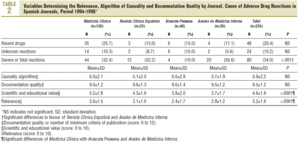
Of the 2244 LE published, 204 (9.1%) related to ADR, which corresponded to 235 cases. The percentage of LE on ADRs was similar for each journal (Table 2). The origins were: hospital in 192 cases (81.7%), 44 (18.7%) in primary care, pharmacy services, 21 (8.9%), 17 (7.2%) pharmacological services, 15 (6.4%) from pharmacovigilance centres and 12 (5.1%) from emergency services. Thirty three cases (14.0%) were notified to PV centres.
The mean age was 53.2±20.3 years, with no difference between sexes and with 53.7% males. There were 85 cases (36.2%) >64 years, 143 (60.9%) adults and 6 (2.6%) <15 years.
Of the 554 drugs (2.4±1.6 per case)administered, 267 (1.1±0.4 per case) of them were implicated. In 24 cases (10.2%) it was due to an interaction between drugs.
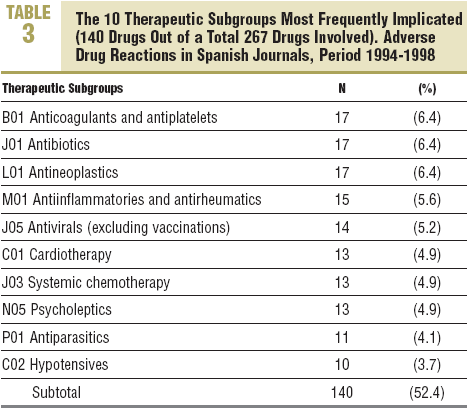
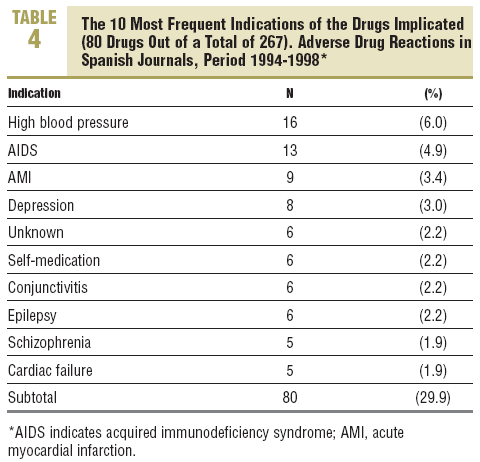
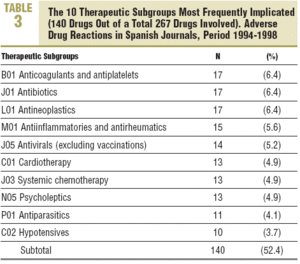
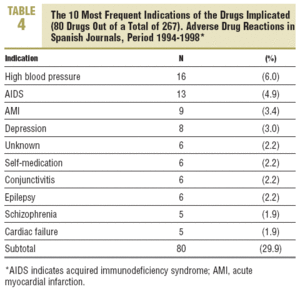
More than 60% of the drugs implicated were concentrated in 5 therapeutic groups: systemic infection therapy (19.9%), nervous system (15%), cardiovascular system (11.2%), blood and haematopoietic organs (9.4%), and digestive system and metabolism (9%). The most frequent subgroups and indications are shown in Tables 3 and 4.
In 48 cases (20.4%) the reaction was due to recent drugs, which in 6 cases were unknown (Table 2).
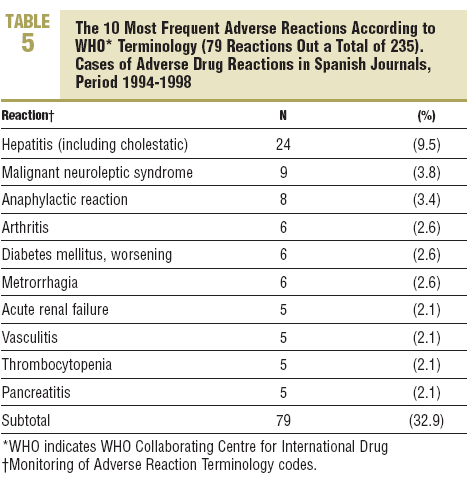
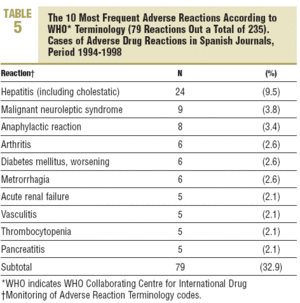
Five groups were involved in more han half of the reactions: the nervous system (13.6%), liver, (10.2%), skin and appendages (9,8%), general (9.8%), and digestive system (8,1%). There was a wide dispersion in the particular ADRs and hepatitis stood out, with 24 cases (9,5%) (Table 5).
As regards causal relationship of the reactions, 3 (1.3%) were improbable, 23 (9.8%) conditional, 64 (27.2%) possible, 117 (49.8%) probable, and 28 (11.9%) definitive. In 168 cases (75%) the ADR was well known beforehand, in 24 (10.2%) unknown and in 43 (18.3%), anecdotal. In 6 cases the drug had to be withdrawn. Thirty two cases (13.6%) were re-exposed to the drug and were positive in 31.
In 118 cases (50.2%) the ADR was moderate, in 67 (28.5%) severe, 37 (15.7%) mild and in 13 cases (5.5%), fatal (Table 2). The severity was similar between sexes and did not increase with age or with the number active ingredients administered.
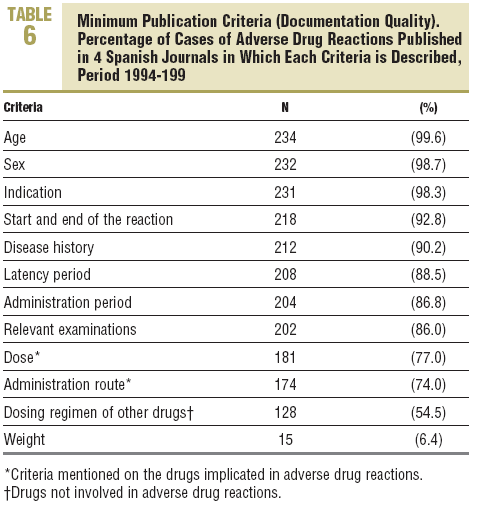
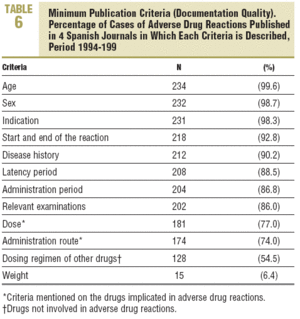
Of the minimum criteria, 9.5±1.2 were recorded (quality of documentation) per case, with no differences between journals (Table 2). Four criteria were recorded in 100% of the cases, 9 or more in 80%, and 4 (1.7%) had the 12 criteria (Table 6). Significant differences were detected in the scientific or educational value and in the relevance of the publication, in favour of Medicina Clínica (Table 2).
Discussion
The importance of publishing suspected ADRs in medical journals is endorsed by the high percentage of previously unknown reactions which are published before being notified to the pharmacovigilance centres.2,15 This explains why Spanish journals dedicated 9.1% of the published LE to the communication of ADR cases during the period studied (1994-1998). This interest in pharmacovigilance has experienced a notable increase in Spanish literature since the period 1972-1974, when articles on any problem on this subject were only 2.1% of the total.9
The majority of published ADRs in Spanish journals come from specialised care (81.7%), with a limited contribution from primary care. However, the family doctor is the professional who traditionally contributes more to notifying, at least in Spain, by yellow card. Also, it is estimated that they see a mean of 2 ADRs per day.16 The simultaneous notification to the PV services (12%) of the cases published is deficient, and could be improved if the journals recommended that they are first communicated to the PV centres.
The predominance of the middle aged group shown in this study agrees with the majority of descriptive studies and PV centres, but not with those from hospital settings, where the elderly predominate.17-21 Likewise, the ratio detected in males and females also contrasts with the majority of PV studies, where females predominate.22
The groups of drugs most frequently involved coincide with data from PV centres.20,23
The percentage of ADR to recent drugs (25.7%), is low and should be improved by the journals which aspire to be the vehicle of authentic new reports which can provide alerts.24 In this sense, a tendency for Medicina Clínica to stand out over the rest of the journals is detected.
The distribution of the reactions by systems generally agrees with data from the PV centres,20,25 although with a higher percentage of hepatic reactions (10.2%); this contrasts with hospital studies, where the most frequent ADRs are normally gastrointestinal due to non-steroidal anti-inflammatories.26,27
The high causal relationship detected, with 77% possible or probable ADRs and 11.9% definitive, was as expected in the scientific journals, since anything else would cause false alarms or scepticism on the part of the readers. A low percentage (10.2%) of previously unknown ADRs were published during the study, broadly coinciding with the cases notified to the PV centres28 and higher than Spanish studies in hospital settings.29 This suggests that, as regards PV, the role of Spanish journals is more one of education or the transfer of new data already reported in higher impact journals to the Spanish scientific community.
If one takes into account that it is hardly ethical to re-expose the patient to the suspect drug, the 13.2% cases which were positive on re-exposure can be considered acceptable and near the 19.2% obtained by Haramburu et al.7
The severity of the ADRs published is an indicator of their importance, therefore it should be desirable for a high percentage of severe reactions to appear in the journals. In the period studied, 34% of ADRs published were severe or fatal, a much higher percentage than that recorded in the Spanish PV centres20 and in other Spanish studies.30
Overall, it can be considered, that in the four journals studied, they have high documentation quality, since they included the majority of the minimum criteria for the publication of an ADR.3-5,8
The results are similar to those shown by Gil et al9 for the period 1992-1994. However, some criteria, such as weight or the dosing regimen of non-involved drugs, are still
lacking; this deficiency would be easy to correct if the journals proposed a list of minimum criteria for the publication of ADRs.
The scientific or educational value, as well as the relevance, is a new concept introduced by Meyboom et al14 which, despite a certain subjectivity, gives added value to the seriousness and documentation quality of the ADRs. In this study, the only journal which was higher than the mean value was Medicina Clínica (5.2 out of 10).
The relevance of the publication was low and Medicina Clínica, although it does not reach the mean value, again stands out against Atención Primaria and Anales de Medicina Interna. This concept of relevance has not been used until now, therefore it has not been possible to make comparisons.14 With its use, it has attempted to go beyond the description of ADRs with the classic categories of attributability by algorithms of causality, an insufficient method in any case to determine the importance of a publication.
In conclusion, it can be stated that the Spanish internal medicine and general medicine journals have a high quality as regards the inclusion of the minimum publication criteria and the causality, although overall their relevance is limited, with none of them reaching a pass mark,
although Medicina Clínica stands out slightly. The cause is mainly due to the fact that the majority of publications
refer to well known reactions, not particularly severe, and reactions to older drugs.
Acknowledgements
The authors would like to thank F.J. Morales-Olivas for his comments and support of this work.