Ozaki et al. first performed aortic valve replacement using autologous pericardium in 2007. Compared to mechanical and bioprosthetic valves which have apparent disadvantages, this technique has been an alternative with a promising safety and efficacy result. A comprehensive search was carried out in 4 databases (Pubmed, Cochrane Library, Proquest, Scopus) from February to March 2021 using search terms “autologous pericardium”, “aortic valve replacement”, and “aortic valve reconstruction”. Outcomes measured in this study were mortality, freedom of operation, thromboembolic and endocarditis event, and echocardiography finding. Risk bias of all studies was measured using MINORS criteria. A total of 12 studies involving 1427 subjects were included. The mean age was 64.95 years and 52.1% subjects were male. Mortality due to cardiac and noncardiac cause was 1.75%. Reoperation was needed in 1.12% subjects. Thromboembolic and endocarditis events occurred in 0.21% and 0.91% respectively. All studies reported lower average peak pressure gradient after surgery. Aortic valve replacement using autologous pericardium has a tolerable safety and efficacy.
Ozaki et al. realizaron por primera vez el reemplazo de la válvula aórtica utilizando pericardio autólogo en 2007. En comparación con las válvulas mecánicas y bioprotésicas, que tienen aparentes desventajas, esta técnica ha sido una alternativa con un resultado prometedor de seguridad y eficacia. Se realizó una búsqueda exhaustiva en 4 bases de datos (Pubmed, Cochrane Library, Proquest y Scopus) de febrero a marzo de 2021 utilizando los términos de búsqueda pericardio autólogo, reemplazo valvular aórtico y reconstrucción valvular aórtica. Los resultados medidos en este estudio fueron mortalidad, libertad de operación, evento tromboembólico y hallazgo ecocardiográfico. El sesgo de riesgo de todos los estudios se midió mediante los criterios MINORS. Se incluyeron un total de 12 estudios con 1.427 sujetos. La edad media fue de 64,95 años y el 52,1% de los sujetos eran varones. La mortalidad por causa cardíaca y no cardíaca fue del 1,75%. Se produjeron episodios tromboembólicos y endocarditis en un 0,21% y un 0,91%, respectivamente. Todos los estudios informaron de un gradiente de presión máxima promedio más bajo después de la cirugía. La sustitución valvular aórtica mediante pericardio autólogo tiene una seguridad y eficacia tolerables.
Aortic valve disease is the most frequent type of valvular disease in Europe and North America, accounting for 2–7% of individuals over 65 years of age. When left untreated, symptom progression is rapid and lethal, with a median survival of less than two years in individuals with heart failure symptoms. Conservative treatment is largely ineffective for the long-term management of aortic valve disease, and aortic valve replacement remains the mainstay of therapy in those with an acceptable risk profile. The perioperative risk of surgical aortic valve replacement among the oldest patients and those with a high burden of comorbidities is often perceived to outweigh the potential long-term benefits, with a perception of high operable risk accounting for 30% of all non-operated patients with symptomatic aortic stenosis.1
The history of treatment for aortic stenosis could be traced back to 1672, in which Rayger firstly recorded the osseous fusion of aortic valve cusps during autopsy. Despite being one of the oldest techniques of open heart surgery, the results of calcified AS after aortic valve repair remain unfavorable. Since the first successful surgical replacements of diseased heart valves were reported in 1960, aortic valve replacement has been the gold standard for the surgical treatment of AS for the past five decades.2
For several decades, pericardial tissue has been widely applied as a good material for valvuloplasty.3 To date, two types of pericardium are available for valvuloplasty: autologous and heterologous. The majority of aortic regurgitation caused by defects in valve leaflets may be successfully treated by replacing the defecting leaflets with autologous or heterologous pericardium.4
The autologous pericardial aortic valve (APAV), molded with the autologous pericardium and treated briefly with glutaraldehyde, was introduced in 1988 by Duran et al.5 Theoretically, this autologous tissue should experience reduced degeneration because of a lack of antigenicity. Moreover, animal studies6–8 and short-term follow-up analyses of several clinical studies5,9 have demonstrated the efficacy and safety of the procedure.
To this date, there are no available systematic reviews that investigate the feasibility of autologous pericardium for aortic valve replacement. The present review aims to determine the safety and efficacy of autologous pericardium for aortic valve replacement.
MethodsThis systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 updated guidelines.
PICOS questionBased on the patient, intervention, comparison, outcome, and study design (PICOS), the question that guided this review was, is the use of autologous pericardium beneficial for adults and elderly undergoing aortic valve replacement?
Search strategySystematic literature searches were carried out in February to March 2021 on PubMed, The Cochrane Library, Scopus, ProQuest using keywords “autologous pericardium”, “aortic valve replacement”, and “aortic valve reconstruction”. The reference lists and bibliographies of included articles were also searched, in addition to hand-searching of various relevant high-impact journals. We also searched on Clinicaltrials.gov to minimize trial left unincluded. The search was restricted to literature written in English and no limitation on publication year.
Outcome measuresThe primary end points included safety (freedom of reoperation, thromboembolic events, endocarditis, and deaths). The secondary outcomes included echocardiographic findings (peak pressure gradient).
Eligibility criteriaInclusion and exclusion criteria are highlighted in Table 1. Two authors independently reviewed title and abstracts to distinguish potentially relevant studies and articles for more extensive review using the inclusion criteria. Eligibility criteria were subsequently applied to the retrieved set of articles by the same authors. Disputes were presented to the third and fourth author.
Data extraction and quality assessmentAn electronic data collection form was developed by two authors. Two authors independently extracted the following data information: first author, publication year, study design, participants, population, methods of aortic valve replacement, other concurrent interventions, main outcomes, and findings. Disagreements were resolved by discussion, or when necessary, adjudicated by a third reviewer. The risk of bias of each study was measured using Methodological Index for Non-Randomized Studies (MINORS) (Methodological index for non-randomized studies (minors) development and validation of a new instrument) The global ideal score was 16 and 24 for non-comparative and comparative studies, respectively.
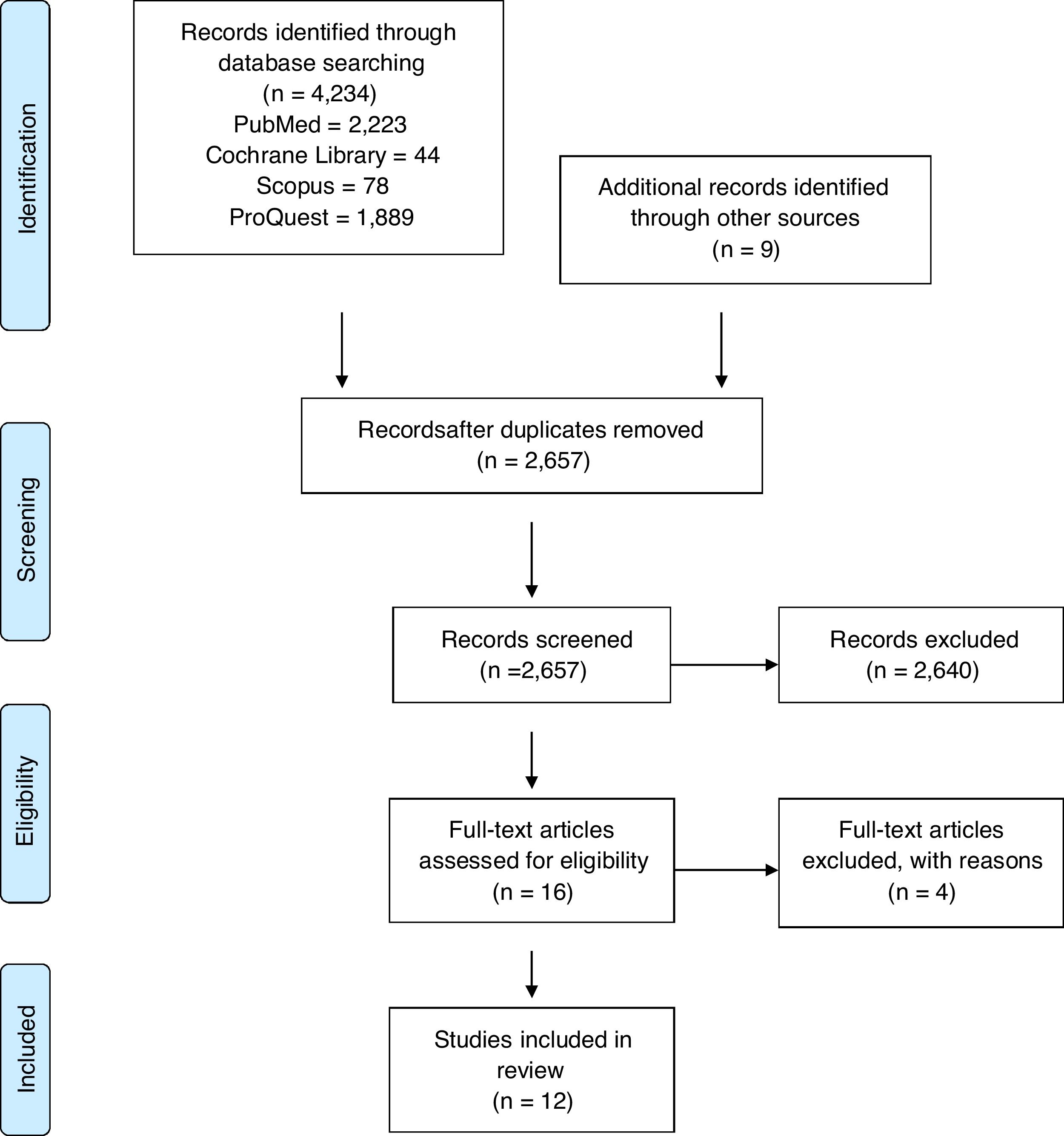
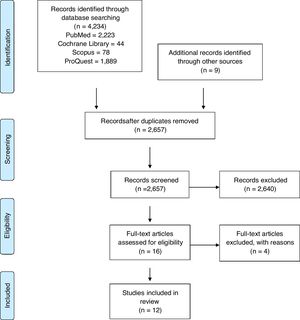
ResultsStudy selectionFrom the 4234 articles initially identified in searches of electronic databases, 12 articles were selected for full-text review after title and abstract review as shown in the PRISMA flow sheet of included studies (Fig. 1).
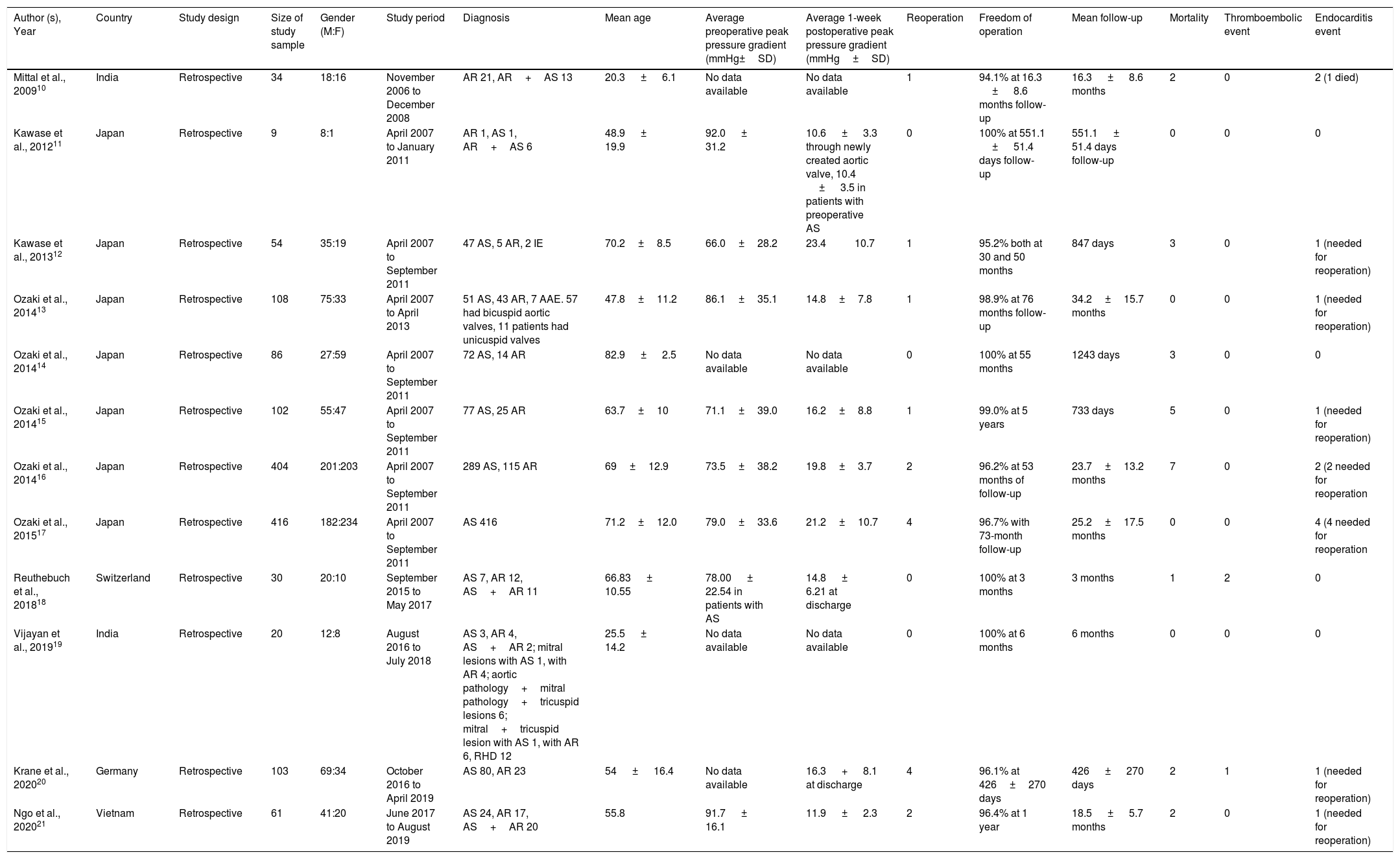
Study characteristicsAll of the included studies were retrospective single-center series. There were 11 observational study and 1 comparative study. No randomized clinical trials were identified in our search. This study involved a total of 1427 patients undergoing aortic valve replacement using glutaraldehyde-treated autologous pericardium. The sample sizes range from 9 to 416; each study was conducted in a single center and published between 2009 and 2020. Characteristics of the included studies are presented in Table 2.
Characteristics of the included studies.
| Author (s), Year | Country | Study design | Size of study sample | Gender (M:F) | Study period | Diagnosis | Mean age | Average preoperative peak pressure gradient (mmHg±SD) | Average 1-week postoperative peak pressure gradient (mmHg±SD) | Reoperation | Freedom of operation | Mean follow-up | Mortality | Thromboembolic event | Endocarditis event |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mittal et al., 200910 | India | Retrospective | 34 | 18:16 | November 2006 to December 2008 | AR 21, AR+AS 13 | 20.3 ± 6.1 | No data available | No data available | 1 | 94.1% at 16.3 ± 8.6 months follow-up | 16.3 ± 8.6 months | 2 | 0 | 2 (1 died) |
| Kawase et al., 201211 | Japan | Retrospective | 9 | 8:1 | April 2007 to January 2011 | AR 1, AS 1, AR+AS 6 | 48.9 ± 19.9 | 92.0 ± 31.2 | 10.6 ± 3.3 through newly created aortic valve, 10.4 ± 3.5 in patients with preoperative AS | 0 | 100% at 551.1 ± 51.4 days follow-up | 551.1 ± 51.4 days follow-up | 0 | 0 | 0 |
| Kawase et al., 201312 | Japan | Retrospective | 54 | 35:19 | April 2007 to September 2011 | 47 AS, 5 AR, 2 IE | 70.2±8.5 | 66.0±28.2 | 23.4 10.7 | 1 | 95.2% both at 30 and 50 months | 847 days | 3 | 0 | 1 (needed for reoperation) |
| Ozaki et al., 201413 | Japan | Retrospective | 108 | 75:33 | April 2007 to April 2013 | 51 AS, 43 AR, 7 AAE. 57 had bicuspid aortic valves, 11 patients had unicuspid valves | 47.8±11.2 | 86.1±35.1 | 14.8±7.8 | 1 | 98.9% at 76 months follow-up | 34.2±15.7 months | 0 | 0 | 1 (needed for reoperation) |
| Ozaki et al., 201414 | Japan | Retrospective | 86 | 27:59 | April 2007 to September 2011 | 72 AS, 14 AR | 82.9 ± 2.5 | No data available | No data available | 0 | 100% at 55 months | 1243 days | 3 | 0 | 0 |
| Ozaki et al., 201415 | Japan | Retrospective | 102 | 55:47 | April 2007 to September 2011 | 77 AS, 25 AR | 63.7±10 | 71.1±39.0 | 16.2±8.8 | 1 | 99.0% at 5 years | 733 days | 5 | 0 | 1 (needed for reoperation) |
| Ozaki et al., 201416 | Japan | Retrospective | 404 | 201:203 | April 2007 to September 2011 | 289 AS, 115 AR | 69±12.9 | 73.5±38.2 | 19.8±3.7 | 2 | 96.2% at 53 months of follow-up | 23.7±13.2 months | 7 | 0 | 2 (2 needed for reoperation |
| Ozaki et al., 201517 | Japan | Retrospective | 416 | 182:234 | April 2007 to September 2011 | AS 416 | 71.2±12.0 | 79.0±33.6 | 21.2±10.7 | 4 | 96.7% with 73-month follow-up | 25.2±17.5 months | 0 | 0 | 4 (4 needed for reoperation |
| Reuthebuch et al., 201818 | Switzerland | Retrospective | 30 | 20:10 | September 2015 to May 2017 | AS 7, AR 12, AS+AR 11 | 66.83 ± 10.55 | 78.00 ± 22.54 in patients with AS | 14.8 ± 6.21 at discharge | 0 | 100% at 3 months | 3 months | 1 | 2 | 0 |
| Vijayan et al., 201919 | India | Retrospective | 20 | 12:8 | August 2016 to July 2018 | AS 3, AR 4, AS+AR 2; mitral lesions with AS 1, with AR 4; aortic pathology+mitral pathology+tricuspid lesions 6; mitral+tricuspid lesion with AS 1, with AR 6, RHD 12 | 25.5 ± 14.2 | No data available | No data available | 0 | 100% at 6 months | 6 months | 0 | 0 | 0 |
| Krane et al., 202020 | Germany | Retrospective | 103 | 69:34 | October 2016 to April 2019 | AS 80, AR 23 | 54 ± 16.4 | No data available | 16.3 + 8.1 at discharge | 4 | 96.1% at 426±270 days | 426 ± 270 days | 2 | 1 | 1 (needed for reoperation) |
| Ngo et al., 202021 | Vietnam | Retrospective | 61 | 41:20 | June 2017 to August 2019 | AS 24, AR 17, AS+AR 20 | 55.8 | 91.7 ± 16.1 | 11.9 ± 2.3 | 2 | 96.4% at 1 year | 18.5 ± 5.7 months | 2 | 0 | 1 (needed for reoperation) |
The MINORS was used to assess the risk of bias in all studies. All observational studies scored 14 while one comparative study scored 24. None of the observational studies reported 95% confidence interval. Of note, all studies were retrospective, which, by definition, are susceptible to major selection bias due to the unknown accuracy of record keeping.
Synthesis of resultsA total of 1427 subjects were included in this study. The mean age was 64.95 years old and 52.1% subjects were male. The diagnosis of preoperative aortic stenosis, aortic regurgitation, and combination of aortic stenosis and aortic regurgitation were 75.40%, 19.62%, and 3.64%, respectively. There were 40 (1.40%) subjects with other diagnosis such as infective endocarditis, pathology with mitral lesion, rheumatic heart disease, and annuloaortic ectasia. All twelve included studies found that those who underwent aortic valve replacement using glutaraldehyde-treated autologous pericardium had adequate safety and efficacy. Of 1427 subjects, 25 (1.75%) died. There were 3 (0.21%) and 13 (0.91%) thromboembolic and endocarditis events that occurred respectively. Reoperation were reported in 16 (1.12%) patients, mainly because of endocarditis (69%). All studies reported reduction of postoperative peak pressure gradient
DiscussionThe origin of aortic valve replacement can be traced back to the early era of cardiac surgery.22 Aortic valve replacement has been performed using native valve cups by various techniques, including commissurotomy, annuloplasty, free edge unrolling, cusp resuspension, supra-aortic crest enhancement, free edge reinforcement, wedge resection, and so on.23 This type of conservative repair is not always possible, particularly for calcified aortic in elderly patients. Simple decalcification or slicing of cusps has not shown good results. On the other hand, there is an apparent limitation of durability with bioprosthetic valves and a tangible disadvantage of anticoagulation with mechanical prosthesis. Moreover, both prostheses cannot create good hemodynamics compared to a native aortic valve.12
Attempts to replace aortic valve cusp tissue with biologic material have been made since the late 1960s.24 Fascia lata, dura mater, and bovine pericardium have been used in a small number of patients. However, the results are not favorable in the majority of instances. Glutaraldehyde-treated autologous pericardium has been used for the aortic valve by Halees et al.25 in their study of aortic valve reconstruction with human pericardium with up to 16 years of follow-up. They found that aortic valve reconstruction was feasible with good hemodynamics, low mortality and thromboembolic events. Furthermore, its behavior at 10 years was comparable to that of stentless aortic valve bioprosthesis.
To the best of our knowledge, this is the first systematic review to determine the safety and efficacy of autologous pericardium for aortic valve replacement. This study has found that glutaraldehyde-treated autologous pericardium has a tolerable safety and efficacy for aortic valve replacement. In the present review, AVR with autologous pericardium was considered safe, with 1.75% mortality. Sixteenth percent of all mortality were caused by cardiac causes including leaflet dehiscence which eventually led to multi organ dysfunction syndrome, endocarditis/paravalvular abscess, cardiac tamponade, and fatal thoracic hemorrhage. Most occurred immediately after surgery or within 1 year after discharge. Noncardiac causes of mortality including pneumonia, cancer, etc. mostly occurred later, years after patients had been discharged. A study of 66,453 subjects studying early mortality after aortic valve replacement with either mechanical or bioprosthetic valve showed operative mortality at 1 year was 7.74% for mechanical valve and 6.11% for bioprosthetic valve.26
There were three (0.21%) thromboembolic events that occurred, which are all postoperative stroke. Thromboembolism after aortic valve reconstruction is the most important valve-related postoperative complication. In fact, it plays a significant role in postoperative morbidity in elderly patients.27 The present review found that the thromboembolic events that occurred in those undergoing AVR with autologous pericardium was lower compared to mechanical or bioprosthetic valves. A meta-analysis28 comparing the outcomes of aortic valve reconstruction using mechanical with biomechanical valves found that the incidence of thromboembolism in the mechanical and bioprosthetic group were 9.8% and 7.96%, respectively.
Endocarditis had to be taken into concern since it was the major cause for reoperation (69%). Also, it caused death in one patient in Mittal et al.10 The use of intravenous antibiotic can resolve the disease and prevent further reoperation. Several studies showed that the incidence of endocarditis and thromboembolic event is still higher in aortic valve reconstruction using autologous pericardium.10,31 Also, it was more difficult to do the reoperation when compared to autologous pericardium for aortic valve replacement.
Conventional aortic valve replacement presents complications derived from prosthetic valves. Mechanical valves require life-long anticoagulation, and bioprosthesis is associated with high rates of degeneration and the need for another revision surgery.29,30 In a study of 1823 patients (mean age, 68.9±10.9 years) undergoing Carpentier-Edwards supra-annular porcine bioprosthesis, Jamieson et al.29 reported that the overall actual cumulative freedom at 18 years from reoperation was 85.0±1.2%. Meanwhile, the need of reoperation in this study was 1.12%. Most were caused by infective endocarditis. The freedom of operation in our included studies were all above 94.1%. At the longest follow-up of 76 months, freedom of reoperation was 98.9%.13
All studies in this review reported improvement of hemodynamic performance 1 week following surgery or at discharge. The average preoperative peak pressure gradient ranged from 66.0±28.2 to 92.0±31.2mmHg, while the postoperative peak pressure gradient ranged from 10.6±3.3 to 23.4±10.7mmHg. Vijayan et al.19 reported significant difference in mean aortic pressure at 6 months follow-up between patient undergoing reconstruction of aortic valve with autologous pericardium and patient undergoing mechanical aortic valve replacement (8.83 [3.66–26.66] mmHg vs 18.16 [6.00–79.67] mmHg, P=0.006). In a study of 154 patients comparing stentless and stented bioprosthesis for aortic valve replacement, peak pressure gradient at 30 days follow-up were 17.0±8.2mmHg and 24.5±9.2mmHg, respectively.31
Human autologous pericardium has been used in several aspects of heart surgery. For instance, it has been used in congenital heart disease for patch repair or right ventricular outflow tract reconstruction. Moreover, it has been used for leaflet extension in mitral valve repair. Chauvaud et al.32 reported valve extension with glutaraldehyde-preserved autologous pericardium in mitral valve repair. In their series, they found no calcification of autologous pericardium in 64 cases with six months to nine years of follow-up.
The present review has several important limitations. Firstly, all of the included studies were nonrandomized and retrospective, leading to an inherent selection bias. Moreover, the sample sizes were small with significant heterogeneity. About 80% of the sample corresponded to the patients published by Ozaki et al. which could be oversized, since it is the same series of patients published by Ozaki et al., between 2012 and 2015, with different subgroups and endpoints. Secondly, the mean follow-up ranged from 3 months to 1243 days (41.4 months). These factors may significantly affect the outcomes of aortic valve replacement, and their effect in the current population for long-term is unknown. Thirdly, only one study had a control group, therefore comparison of the pooled results was mostly limited to historical outcomes of conventional aortic valve replacement.
ConclusionIn conclusion, aortic valve replacement with a glutaraldehyde-treated autologous pericardium can be safely performed, with excellent survival, a low rate of reoperation at short- to middle-term follow-up, and good hemodynamic improvement. Nevertheless, further studies with longer follow-up period are required to investigate the long-term freedom of operation and comparison with mechanical and bioprosthetic valve.
Conflict of interestThe authors declare that they have no conflict of interest.