Inflammatory pseudotumors are considered reactive tumor lesions that are benign in nature and show a spectrum of nonspecific inflammatory or fibrotic reparative changes from a histologic standpoint. The term inflammatory pseudotumor covers a wide range of pathologic processes, including postoperative reparative lesions, tumors like dendritic cell and other more aggressive neoplasms, including inflammatory myofibroblastic tumors.1,2 Although their origin is unknown, possible causes include infectious agents, such as the Epstein–Barr virus.3–5
We present the case of a 43-year-old woman with a history of myasthenia gravis, migraine and infectious mononucleosis one year before, confirmed by serology. In the study of the myasthenia episode in 2010, a splenic lesion was identified and studied.
The patient did not report any abdominal symptoms and physical examination was normal.
Lab work-up showed a high sedimentation rate (1st hour: 52mm). Protein electrophoresis was normal, and immunoglobulin study detected elevated IgG (1930mg/dL) and IgA (414mg/dL) levels. The microbiological study included serology for hydatidosis, hepatitis (A, B and C), human immunodeficiency virus 1 and 2, Brucella, Mycoplasma, herpes, cytomegalovirus and toxoplasma, all of which were negative. Epstein–Barr virus serology was positive for IgG anti-VCA and IgG anti-EBNA, and negative for IgM anti-VCA. Tumor marker levels were normal.
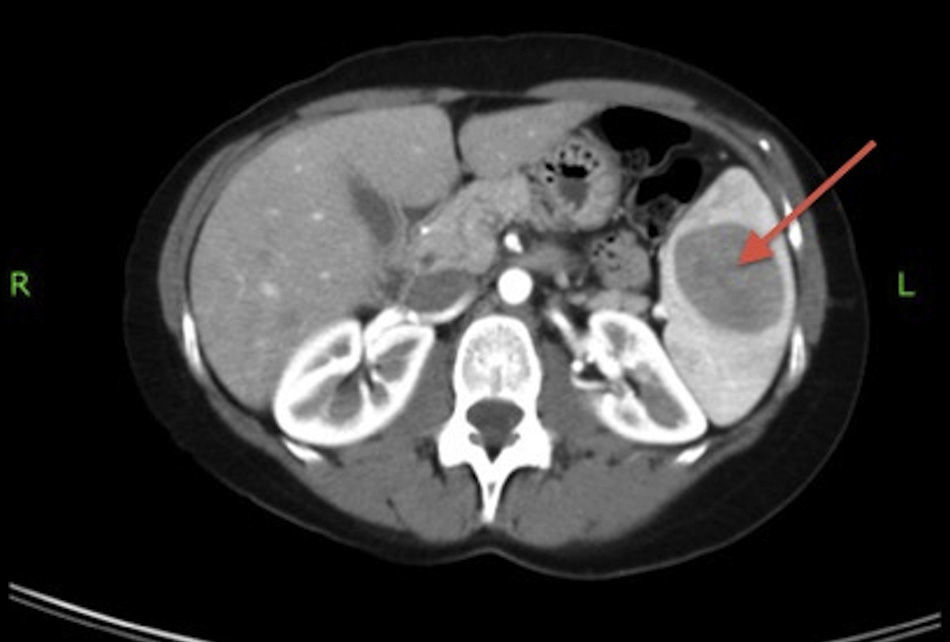
Abdominal CT with intravenous contrast showed a single lesion measuring 39×41mm located in the upper spleen, with nonspecific characteristics. The differential diagnosis was established with atypical hemangioma, hamartoma, fibroma or lymphoma (Fig. 1).
MRI confirmed a normal-sized spleen with a 4cm solid lesion that, according to the image, could be a hemangioma, hamartoma or, less likely, lymphoma.
Scintigraphy with marked erythrocytes showed an area of low uptake in the spleen, ruling out splenic angioma.
As it was impossible to reach an accurate diagnosis, we decided to operate. A whitish lesion was observed at the upper splenic pole, and a laparotomic splenectomy was performed because our hospital had no experience in the laparoscopic approach of this condition. The patient progressed satisfactorily and was discharged after 4 days.
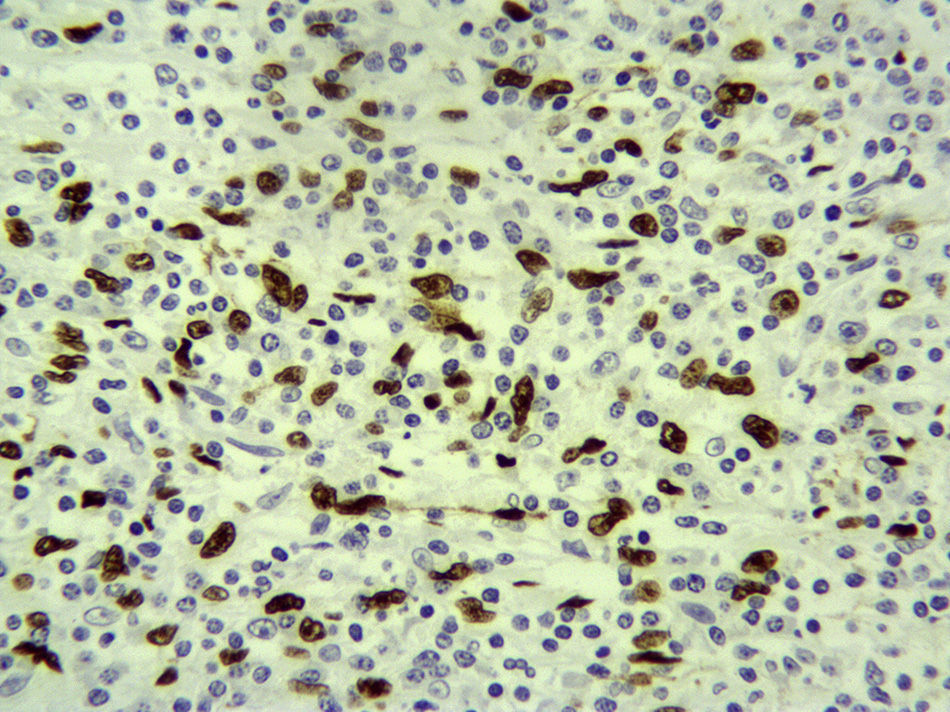
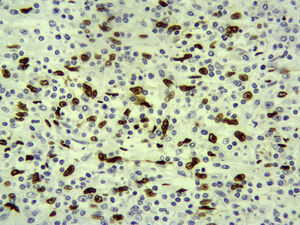
The pathology results showed the splenic tissue had altered architecture due to a proliferation of spindle cells that were thin, homogenous and in short bundles, accompanied by an inflammatory infiltrate formed by lymphocytes, more T than B, polytypical plasma cells, macrophages and some eosinophils. The fusiform cells were positive for pan-cytokeratin, CK18, and actin, while many of them had a strong nuclear positivity for Epstein–Barr virus-encoded RNA (EBER). There was no p53 or ALK expression. The proliferation rate was low in the spindle cells. All of the above was compatible with inflammatory myofibroblastic tumor (inflammatory pseudotumor) (Fig. 2).
The patient continues to be asymptomatic to date.
This type of tumor was described for the first time in the lungs by Brunn in 1939. Later cases reported different locations, such as the lymph nodes, digestive tract and liver.6 The localization of these tumors in the spleen, however, is very uncommon, and few cases have been published since the first report by Cotelingam and Jaffe in 1984.7 The presentation is most frequent in the 2nd and 3rd decades of life, with no predilection for either sex.1
The etiology is unknown, although several theories have been proposed, including: infectious agents, especially Epstein–Barr virus3–5; vascular origin, based on the presence in the lesion of dilated vascular structures and thrombi; and, lastly, autoimmune processes. This latter etiological theory is based on reports of cases in association with several autoimmune disorders, such as idiopathic thrombocytopenic purpura.8
In the case described in this article, the Epstein–Barr studies of the splenectomy specimen were positive. The patient had a history of infectious mononucleosis one year before (confirmed by serology), which was probably involved in the pathogenesis of this case.
The presentation of these inflammatory pseudotumors can be asymptomatic, as in our case. In those cases with associated symptoms, the clinical manifestations are totally nonspecific.
As for laboratory studies, alterations may be seen in hematology tests (mainly anemia and thrombocytopenia), related with splenic sequestration, and there are cases associated with autoimmune thrombocytopenic purpura.8
Diagnostic suspicion is based on radiology studies. Ultrasound, CT and MRI are all useful, although there are no conclusive radiological findings that provide a definitive diagnosis. The differential diagnosis is established with several pathologies, mainly with lymphoproliferative processes and hematological neoplasms. Together with these entities, the differential diagnosis should include other splenic lesions, such as hamartomas, parasitic cysts, those of epithelial or trauma origin, and different granulomatous diseases, such as sarcoidosis.1,9 When it is impossible to reach a definitive diagnosis, surgery is usually indicated, after which there have been no reports of recurrence or the development of other hematologic neoplasms. In recent cases, radio-guided biopsy has provided the definitive diagnosis.10 Thus, it could be said that the diagnosis of inflammatory pseudotumor is, essentially, histologic.
Please cite this article as: Palomeque Jiménez A, Reyes Moreno M, Calzado Baeza S, Ranea Jimena SA, Robayo Soto PS. Detección del virus de Epstein-Barr en un tumor miofibroblástico inflamatorio de bazo (seudotumor inflamatorio). Cir Esp. 2015;93:e41–e43.