Duodenal adenocarcinoma is a rare malignancy. Given the rarity of the disease, there is limited data related to resection results. The objective is to analyze results at our hospital after the curative resection of duodenal adenocarcinoma (DA).
Material and methodsThe variables were retrospectively collected from patients operated on between 1990 and 2017 at our hospital.
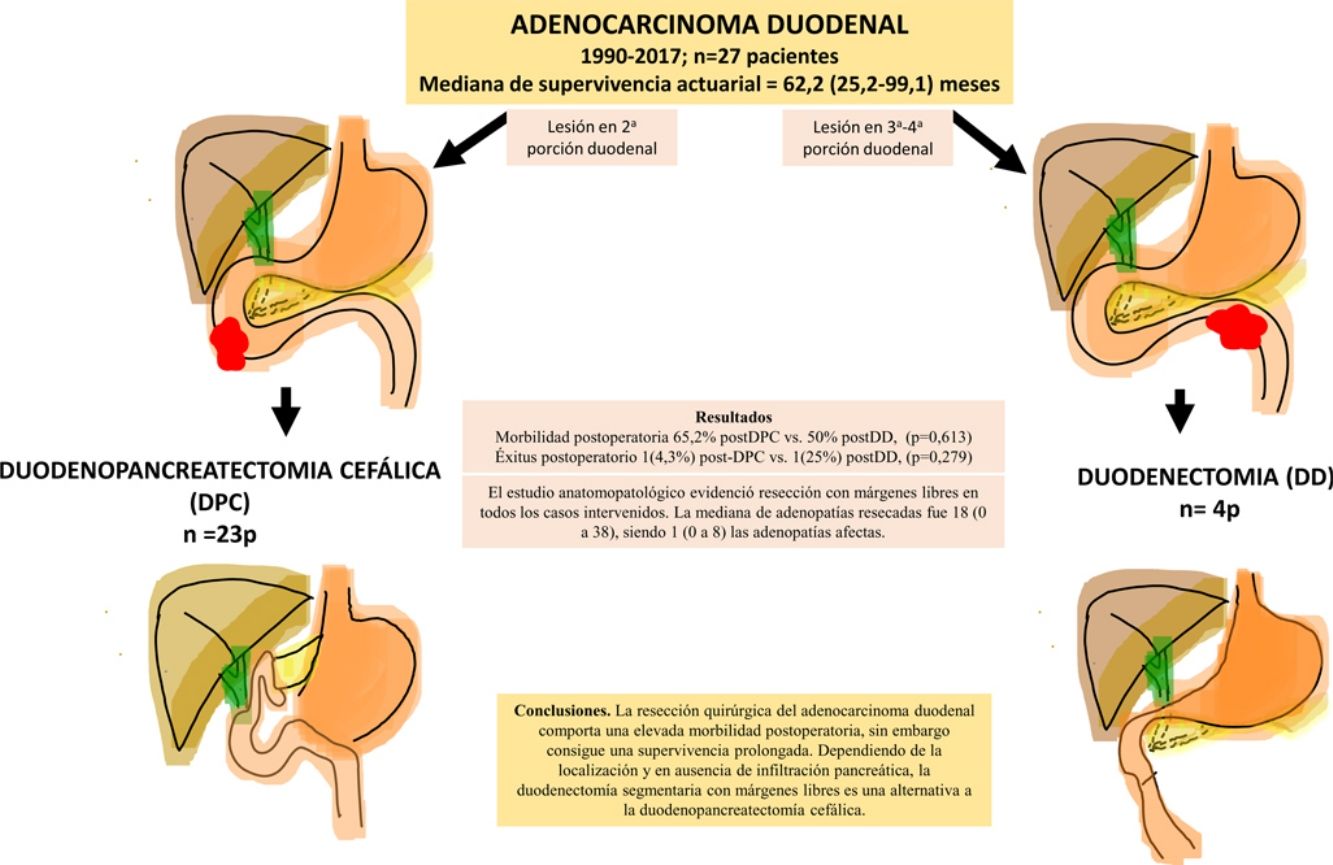
ResultsA total of 27 patients were treated. Twenty-three patients (85%) underwent pancreaticoduodenectomy, and 4 patients (15%) with tumors located in the third and fourth portions of the duodenum underwent segmental duodenal resection. The overall postoperative morbidity was 63% (17 patients). Postoperative mortality was 7% (2 patients); however, postoperative mortality related to surgery was 4% (1 patient). All patients had negative resection margins. A median of 18 lymph nodes (range, 0–38) were retrieved and evaluated, with a median of 1 involved node (range, 0–8). Median follow up was 23 (9–69.7) months. Actuarial overall survival was 62.2 (25.2–99.1) months. Actuarial disease-free survival was 49 (0–133) months.
ConclusionsThe surgical treatment of duodenal adenocarcinoma is associated with a high morbidity, although it achieves considerable survival. Depending on the tumor location and if there is no pancreatic infiltration, segmental duodenal resection with negative margins is an alternative to cephalic pancreaticoduodenectomy.
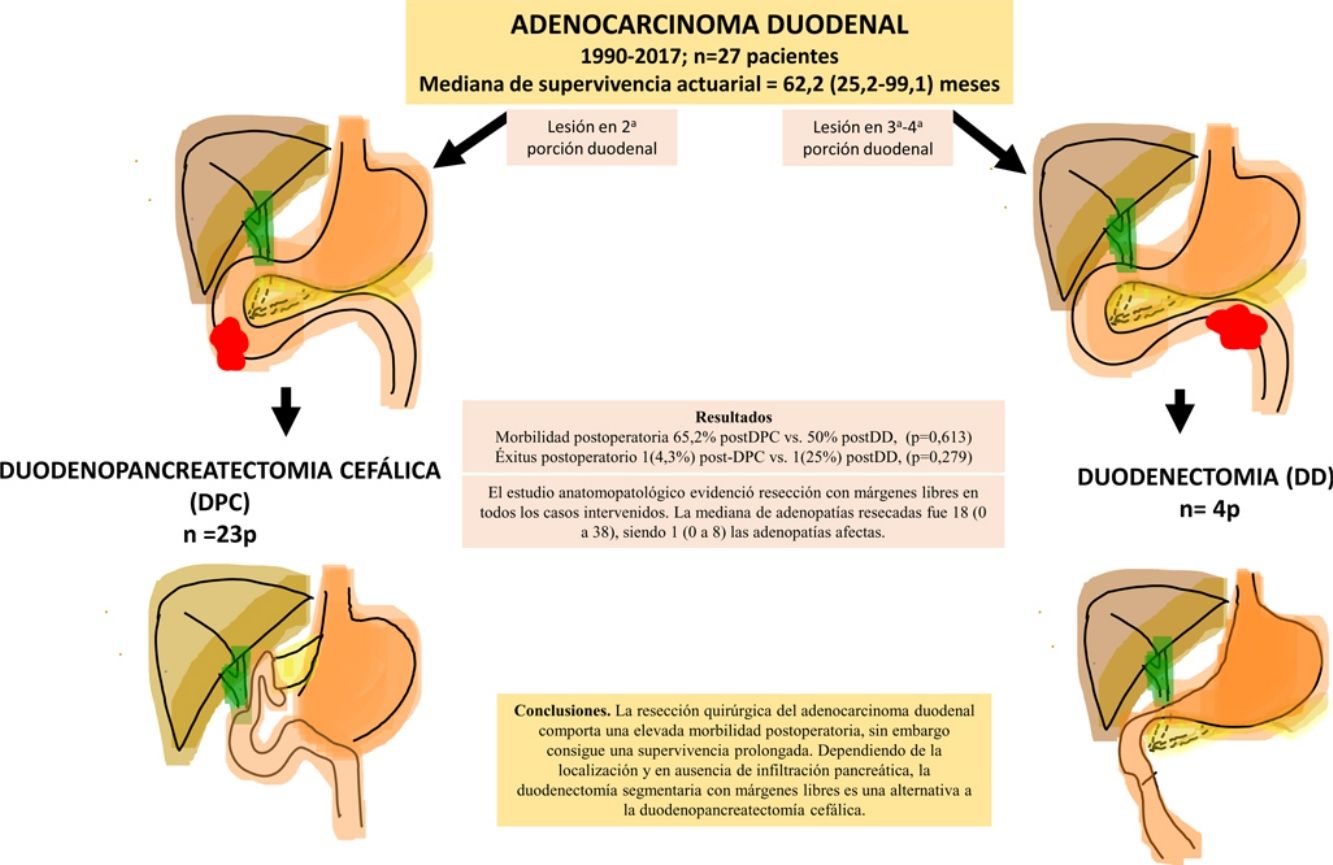
El adenocarcinoma de duodeno es una neoplasia poco frecuente, sobre la que existen pocas experiencias publicadas de los resultados tras su resección. El objetivo es analizar los resultados obtenidos en nuestro centro tras la resección curativa del adenocarcinoma duodenal (AD).
Material y métodosEstudio retrospectivo de los pacientes intervenidos con resección curativa por AD entre 1990 y 2017 en nuestro hospital.
ResultadosSe intervinieron 27 pacientes. En 23 casos (85%) se realizó duodenopancreatectomía cefálica (DPC) y en 4 casos (15%) con localización en 3ª-4ª porción duodenal se realizó duodenectomía segmentaria (DD). La morbilidad postoperatoria global fue del 63% (17 pacientes). La mortalidad postoperatoria global fue 7% (2 pacientes), sin embargo, la mortalidad postoperatoria relacionada con la cirugía fue de 4% (1 paciente). El estudio anatomopatológico evidenció resección con márgenes libres en todos los casos intervenidos. La mediana de adenopatías resecadas fue 18 (0 a 38), siendo 1 (0 a 8) las adenopatías afectas. Tras una mediana de seguimiento de 23 (9–69,7) meses, la supervivencia actuarial fue de 62,2 (25,2 a 99,1) meses y la supervivencia actuarial libre de enfermedad fue de 49 (0 a 133) meses.
ConclusionesLa resección quirúrgica del adenocarcinoma duodenal comporta una elevada morbilidad postoperatoria, sin embargo, consigue una supervivencia prolongada. Dependiendo de la localización y en ausencia de infiltración pancreática, la duodenectomía segmentaria con márgenes libres es una alternativa a la duodenopancreatectomía cefálica.
Duodenal adenocarcinoma (DA) is an uncommon neoplasm, even though the duodenum is the most common location for adenocarcinomas of the small intestine.1–4 The majority of DA are located in the second part of the duodenum (D2), followed by the 3rd and 4th parts (D3-D4), while the first part is very rarely affected.3 The symptoms are nonspecific, such as abdominal pain, nausea, vomiting, asthenia or weight loss, and abdominal pain is the most frequent. In cases of advanced disease, jaundice, intestinal obstruction or anemia may occur. Due to the non-specificity of the symptoms, the diagnosis of the disease is usually late.
The suspected diagnosis is confirmed by upper gastrointestinal endoscopy with biopsy. Multislice abdominal computed tomography scan is essential for the extension study.5 The treatment of choice is surgical resection. Regarding the technique, initially pancreaticoduodenectomy (PD) was considered standard treatment.6 Afterwards, segmental resection or surgical duodenectomy was considered a valid option for this type of tumors, (DD), depending on the location, resection margins, surgical lymphadenectomy.4,7–9 However, there is no consensus in this regard, some authors support PD|as, the technique of choice in all cases.10,11 The objective of this study is to analyze the results obtained at our hospital after the curative resection of DA using PD, DD.
MethodsBetween 1990 and 2017, we retrospectively registered patients who underwent surgery with curative intent for DA at the Hospital Universitari de Bellvitge (Barcelona, Spain). Demographic, clinical, surgical and pathological variables were collected, as well as data regarding the postoperative and long-term evolution. The inclusion period ended in March 2017. The patients were monitored in outpatient surgery consultations, and follow-up was completed in March 2018.
Preoperative StudyAll patients were studied using upper gastrointestinal endoscopy with biopsy and computed tomography. The presence of distant metastases and arterial invasion (superior mesenteric artery, hepatic artery or celiac trunk) were defined as unresectable criteria. Regarding venous involvement, no resections were performed in patients with large duodenal tumors that would involve obliteration of the superior mesenteric vein. Neither advanced patient age nor tumor size were contraindications for surgery.
Surgical Technique and Pathological StudyThe surgical techniques used were PD in those cases in which the tumor affected D2 or there was invasion of the pancreas, and DD for patients with D3-D4 involvement. In tumors that were treated with PD due to their location, standard locoregional lymphadenectomy was performed (peripancreatic lymphadenopathies, hepatic hilum and right margin of the superior mesenteric artery). The pathological study analyzed the degree of tumor differentiation, tumor size, involvement of resection margins, number of affected and resected lymphadenopathies and established the TNM stage (7th Edition of the American Joint Committee on Cancer [AJCC] Staging system for small bowel adenocarcinoma12). In both the DD and PD, the intestinal margin was considered a resection margin. The lymph node ratio was defined as the number of lymph nodes affected over the total number of resected lymph nodes.
Postoperative MorbidityPostoperative morbidity was registered in accordance with the Clavien-Dindo classification.13 Postoperative mortality was defined as death that occurred during hospitalization or up to 90 days after surgery. Delayed gastric emptying (DGE) was defined as the inability to tolerate oral intake after the first postoperative week, following the definition of the International Study Group of Pancreatic Surgery (ISGPS).14 Pancreatic fistula was defined as discharge of fluid through the drain tubes that was rich in amylase (amylase ×3 compared to normal blood levels) after postoperative day 3, applying the ISGPS classification.15 All patients were followed by a member of the surgical team after hospital discharge.
Statistical AnalysisThe Stata v.13® statistical package was used. Initially, a descriptive analysis of the series was performed using measures of central tendency (median) and dispersion (interquartile range and range) for quantitative variables. Next, the series was divided into 2 study groups according to the technique used, PD and DD, and the 2 groups were compared using Fisher’s test for qualitative variables and the Mann–Whitney U for quantitative variables. A P level <.05 was considered statistically significant. Actuarial survival rates (global and disease-free) were calculated for each study group using the Kaplan–Meier test. A study of risk factors for mortality and recurrence was conducted with univariate Cox regression models. For the multivariate analysis, we selected the significant variables from the univariate study, the study variable (surgical technique), as well as the demographic variables (age and sex).
ResultsDuring the study period, 27 patients were treated surgically. In 23 cases (85%), PD was conducted, and in 4 cases (15%) DD was performed (Table 1). The median patient age was 63.4 (50.8–74.1) years, with no significant differences between the 2 groups. The patients included 13 women (48%): 11 in the PD group (48%) and 2 in the DD group (50%).
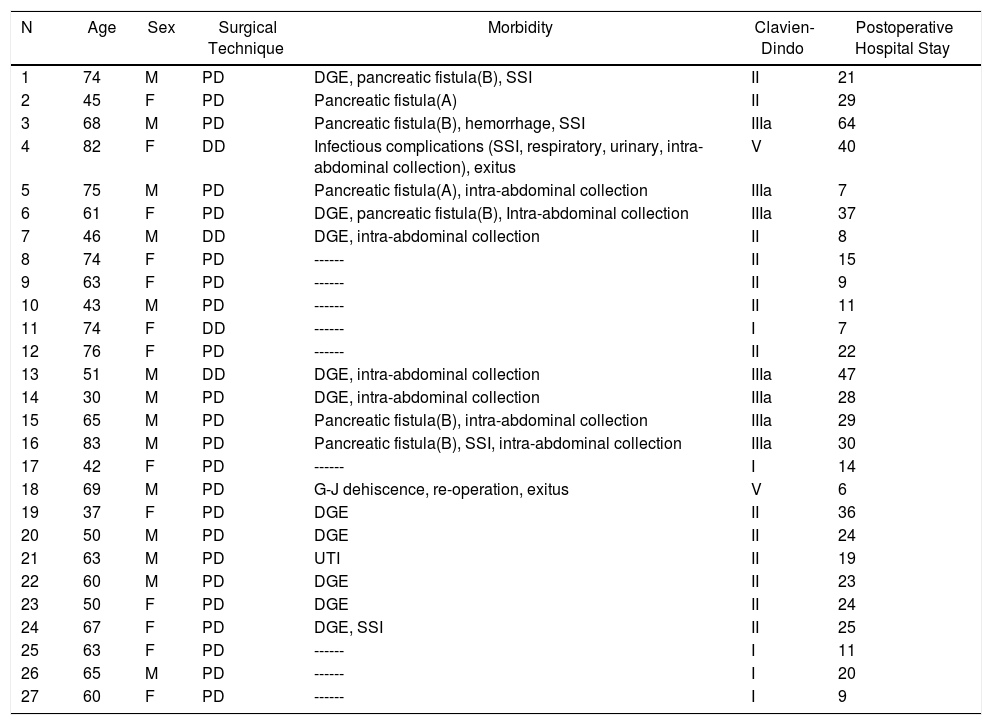
Duodenal Adenocarcinoma: Results of Surgical Treatment, Details of Surgical Technique and Postoperative Morbidity.
| N | Age | Sex | Surgical Technique | Morbidity | Clavien-Dindo | Postoperative Hospital Stay |
|---|---|---|---|---|---|---|
| 1 | 74 | M | PD | DGE, pancreatic fistula(B), SSI | II | 21 |
| 2 | 45 | F | PD | Pancreatic fistula(A) | II | 29 |
| 3 | 68 | M | PD | Pancreatic fistula(B), hemorrhage, SSI | IIIa | 64 |
| 4 | 82 | F | DD | Infectious complications (SSI, respiratory, urinary, intra-abdominal collection), exitus | V | 40 |
| 5 | 75 | M | PD | Pancreatic fistula(A), intra-abdominal collection | IIIa | 7 |
| 6 | 61 | F | PD | DGE, pancreatic fistula(B), Intra-abdominal collection | IIIa | 37 |
| 7 | 46 | M | DD | DGE, intra-abdominal collection | II | 8 |
| 8 | 74 | F | PD | ------ | II | 15 |
| 9 | 63 | F | PD | ------ | II | 9 |
| 10 | 43 | M | PD | ------ | II | 11 |
| 11 | 74 | F | DD | ------ | I | 7 |
| 12 | 76 | F | PD | ------ | II | 22 |
| 13 | 51 | M | DD | DGE, intra-abdominal collection | IIIa | 47 |
| 14 | 30 | M | PD | DGE, intra-abdominal collection | IIIa | 28 |
| 15 | 65 | M | PD | Pancreatic fistula(B), intra-abdominal collection | IIIa | 29 |
| 16 | 83 | M | PD | Pancreatic fistula(B), SSI, intra-abdominal collection | IIIa | 30 |
| 17 | 42 | F | PD | ------ | I | 14 |
| 18 | 69 | M | PD | G-J dehiscence, re-operation, exitus | V | 6 |
| 19 | 37 | F | PD | DGE | II | 36 |
| 20 | 50 | M | PD | DGE | II | 24 |
| 21 | 63 | M | PD | UTI | II | 19 |
| 22 | 60 | M | PD | DGE | II | 23 |
| 23 | 50 | F | PD | DGE | II | 24 |
| 24 | 67 | F | PD | DGE, SSI | II | 25 |
| 25 | 63 | F | PD | ------ | I | 11 |
| 26 | 65 | M | PD | ------ | I | 20 |
| 27 | 60 | F | PD | ------ | I | 9 |
DD: duodenectomy; PD: pancreaticoduodenectomy; F: female; SSI: surgical site infection; UTI: urinary tract infection; M: male; DGE: delayed gastric emptying.
Morbidity according to Clavien-Dindo classification30; hospital stay in days.
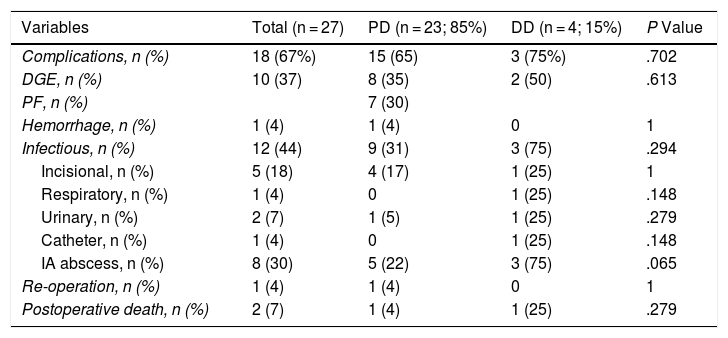
The overall postoperative morbidity was 63% (17 patients). Three patients (11%) presented a complication higher than IIIa of the Clavien–Dindo classification.13 When we analyzed the complications in terms of the technique performed, postoperative morbidity was 65% after PD (15 out of 23 patients) and 75% after DD (3 out of 4 patients), with no statistically significant differences (Table 2). The most frequent complication in the entire series was DGE, which was observed in 37% (10 patients), with no statistically significant differences between the two techniques. Septic complications were seen in 44% (12 patients). Finally, the pancreatic fistula rate after PD was 30% (7 patients: 2 type A and 5 type B). The median postoperative stay was 22 (11–29) days, which was similar in both groups. Out of the total of the series, one patient (4%) had to be reoperated due to dehiscence of the gastrojejunal anastomosis, requiring sutures and surgical drain placement. Postoperative mortality was 7% (2 patients). One patient of the PD group died due to multiple organ failure after being reoperated for anastomotic dehiscence. Another patient of the DD group died 40 days after surgery due to hyperammonemic encephalopathy and respiratory failure, without having presented any intra-abdominal complications. There were no statistically significant differences in postoperative mortality between the two study groups (P = .279) (Table 2).
.Postoperative Morbidity: Comparison Between the 2 Study Groups.
| Variables | Total (n = 27) | PD (n = 23; 85%) | DD (n = 4; 15%) | P Value |
|---|---|---|---|---|
| Complications, n (%) | 18 (67%) | 15 (65) | 3 (75%) | .702 |
| DGE, n (%) | 10 (37) | 8 (35) | 2 (50) | .613 |
| PF, n (%) | 7 (30) | |||
| Hemorrhage, n (%) | 1 (4) | 1 (4) | 0 | 1 |
| Infectious, n (%) | 12 (44) | 9 (31) | 3 (75) | .294 |
| Incisional, n (%) | 5 (18) | 4 (17) | 1 (25) | 1 |
| Respiratory, n (%) | 1 (4) | 0 | 1 (25) | .148 |
| Urinary, n (%) | 2 (7) | 1 (5) | 1 (25) | .279 |
| Catheter, n (%) | 1 (4) | 0 | 1 (25) | .148 |
| IA abscess, n (%) | 8 (30) | 5 (22) | 3 (75) | .065 |
| Re-operation, n (%) | 1 (4) | 1 (4) | 0 | 1 |
| Postoperative death, n (%) | 2 (7) | 1 (4) | 1 (25) | .279 |
DD: duodenectomy; PD: pancreaticoduodenectomy; IA: intra-abdominal; PF: pancreatic fistula; DGE: delayed gastric emptying.
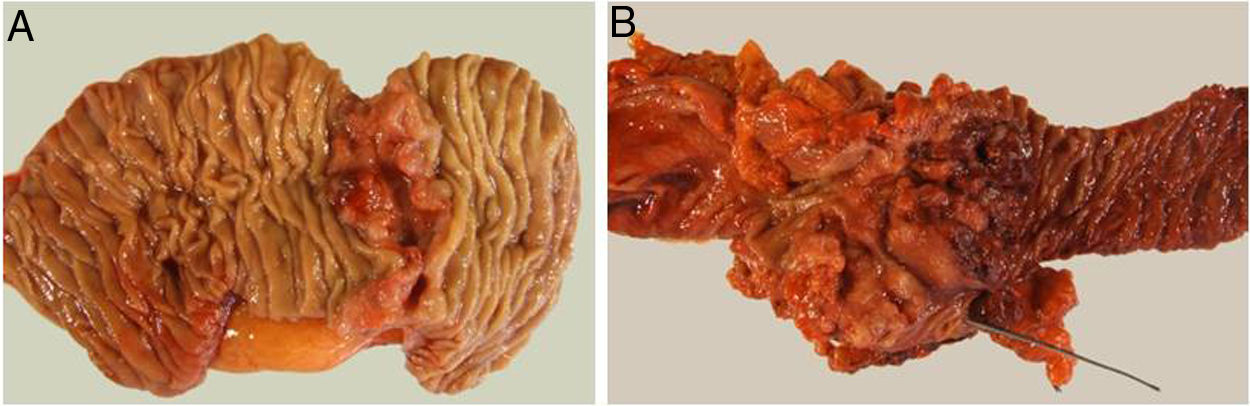
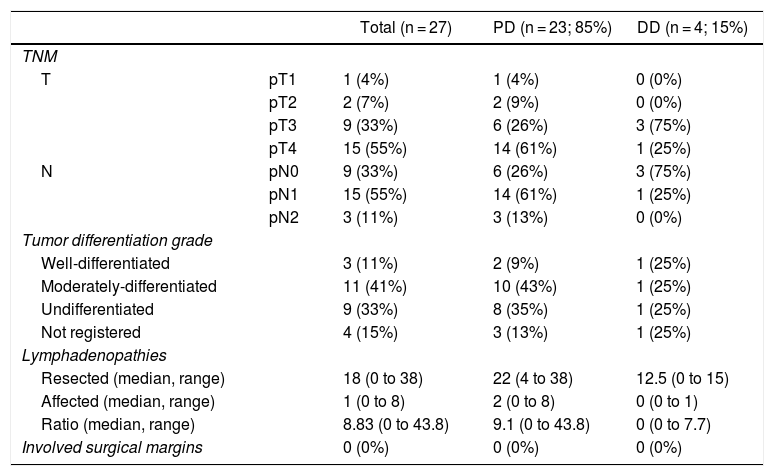
The pathological study showed that the majority of patients were pT4 (15 patients, 55%), with pN1 lymph node involvement (15 patients, 55%) according to the TNM classification (7th edition of the AJCC12) (Tables 3 and 4) (Fig. 1). In 4 patients, the pathological study could not be fully recovered. The degree of tumor differentiation most frequently registered was moderately differentiated (41%). Tumor size was greater than 30 mm in 59% (16 cases). In all cases, resection was performed with free margins. The median number of resected lymphadenopathies was 18 (0–38), including 22 in the PD group (4–38) and 12.5 (0–15) in the DD group, which were statistically significant differences (P = .016).
Pathologic Anatomy Results.
| Total (n = 27) | PD (n = 23; 85%) | DD (n = 4; 15%) | ||
|---|---|---|---|---|
| TNM | ||||
| T | pT1 | 1 (4%) | 1 (4%) | 0 (0%) |
| pT2 | 2 (7%) | 2 (9%) | 0 (0%) | |
| pT3 | 9 (33%) | 6 (26%) | 3 (75%) | |
| pT4 | 15 (55%) | 14 (61%) | 1 (25%) | |
| N | pN0 | 9 (33%) | 6 (26%) | 3 (75%) |
| pN1 | 15 (55%) | 14 (61%) | 1 (25%) | |
| pN2 | 3 (11%) | 3 (13%) | 0 (0%) | |
| Tumor differentiation grade | ||||
| Well-differentiated | 3 (11%) | 2 (9%) | 1 (25%) | |
| Moderately-differentiated | 11 (41%) | 10 (43%) | 1 (25%) | |
| Undifferentiated | 9 (33%) | 8 (35%) | 1 (25%) | |
| Not registered | 4 (15%) | 3 (13%) | 1 (25%) | |
| Lymphadenopathies | ||||
| Resected (median, range) | 18 (0 to 38) | 22 (4 to 38) | 12.5 (0 to 15) | |
| Affected (median, range) | 1 (0 to 8) | 2 (0 to 8) | 0 (0 to 1) | |
| Ratio (median, range) | 8.83 (0 to 43.8) | 9.1 (0 to 43.8) | 0 (0 to 7.7) | |
| Involved surgical margins | 0 (0%) | 0 (0%) | 0 (0%) | |
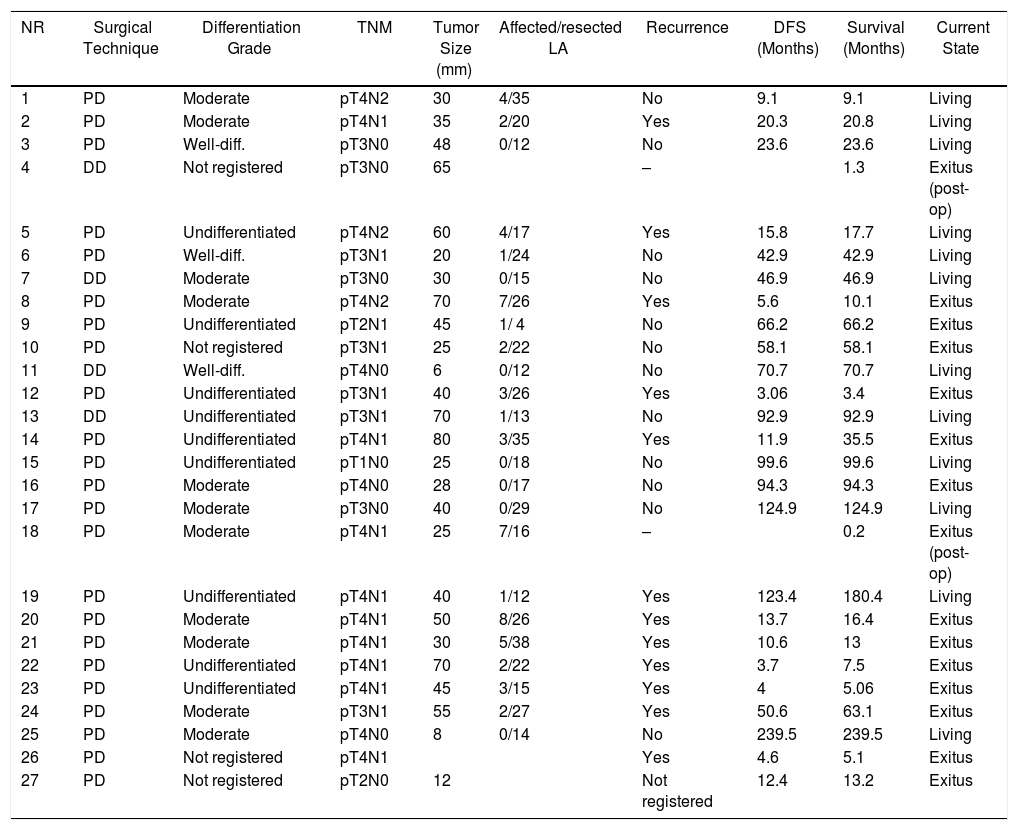
Duodenal Adenocarcinoma: Surgical Treatment Results, Pathology Study and Long-term Evolution.
| NR | Surgical Technique | Differentiation Grade | TNM | Tumor Size (mm) | Affected/resected LA | Recurrence | DFS (Months) | Survival (Months) | Current State |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PD | Moderate | pT4N2 | 30 | 4/35 | No | 9.1 | 9.1 | Living |
| 2 | PD | Moderate | pT4N1 | 35 | 2/20 | Yes | 20.3 | 20.8 | Living |
| 3 | PD | Well-diff. | pT3N0 | 48 | 0/12 | No | 23.6 | 23.6 | Living |
| 4 | DD | Not registered | pT3N0 | 65 | – | 1.3 | Exitus (post-op) | ||
| 5 | PD | Undifferentiated | pT4N2 | 60 | 4/17 | Yes | 15.8 | 17.7 | Living |
| 6 | PD | Well-diff. | pT3N1 | 20 | 1/24 | No | 42.9 | 42.9 | Living |
| 7 | DD | Moderate | pT3N0 | 30 | 0/15 | No | 46.9 | 46.9 | Living |
| 8 | PD | Moderate | pT4N2 | 70 | 7/26 | Yes | 5.6 | 10.1 | Exitus |
| 9 | PD | Undifferentiated | pT2N1 | 45 | 1/ 4 | No | 66.2 | 66.2 | Exitus |
| 10 | PD | Not registered | pT3N1 | 25 | 2/22 | No | 58.1 | 58.1 | Exitus |
| 11 | DD | Well-diff. | pT4N0 | 6 | 0/12 | No | 70.7 | 70.7 | Living |
| 12 | PD | Undifferentiated | pT3N1 | 40 | 3/26 | Yes | 3.06 | 3.4 | Exitus |
| 13 | DD | Undifferentiated | pT3N1 | 70 | 1/13 | No | 92.9 | 92.9 | Living |
| 14 | PD | Undifferentiated | pT4N1 | 80 | 3/35 | Yes | 11.9 | 35.5 | Exitus |
| 15 | PD | Undifferentiated | pT1N0 | 25 | 0/18 | No | 99.6 | 99.6 | Living |
| 16 | PD | Moderate | pT4N0 | 28 | 0/17 | No | 94.3 | 94.3 | Exitus |
| 17 | PD | Moderate | pT3N0 | 40 | 0/29 | No | 124.9 | 124.9 | Living |
| 18 | PD | Moderate | pT4N1 | 25 | 7/16 | – | 0.2 | Exitus (post-op) | |
| 19 | PD | Undifferentiated | pT4N1 | 40 | 1/12 | Yes | 123.4 | 180.4 | Living |
| 20 | PD | Moderate | pT4N1 | 50 | 8/26 | Yes | 13.7 | 16.4 | Exitus |
| 21 | PD | Moderate | pT4N1 | 30 | 5/38 | Yes | 10.6 | 13 | Exitus |
| 22 | PD | Undifferentiated | pT4N1 | 70 | 2/22 | Yes | 3.7 | 7.5 | Exitus |
| 23 | PD | Undifferentiated | pT4N1 | 45 | 3/15 | Yes | 4 | 5.06 | Exitus |
| 24 | PD | Moderate | pT3N1 | 55 | 2/27 | Yes | 50.6 | 63.1 | Exitus |
| 25 | PD | Moderate | pT4N0 | 8 | 0/14 | No | 239.5 | 239.5 | Living |
| 26 | PD | Not registered | pT4N1 | Yes | 4.6 | 5.1 | Exitus | ||
| 27 | PD | Not registered | pT2N0 | 12 | Not registered | 12.4 | 13.2 | Exitus |
Well-diff.: well-differentiated; DD: duodenectomy; PD: pancreaticoduodenectomy; DFS: disease-free survival, LA: lymphadenopathies.
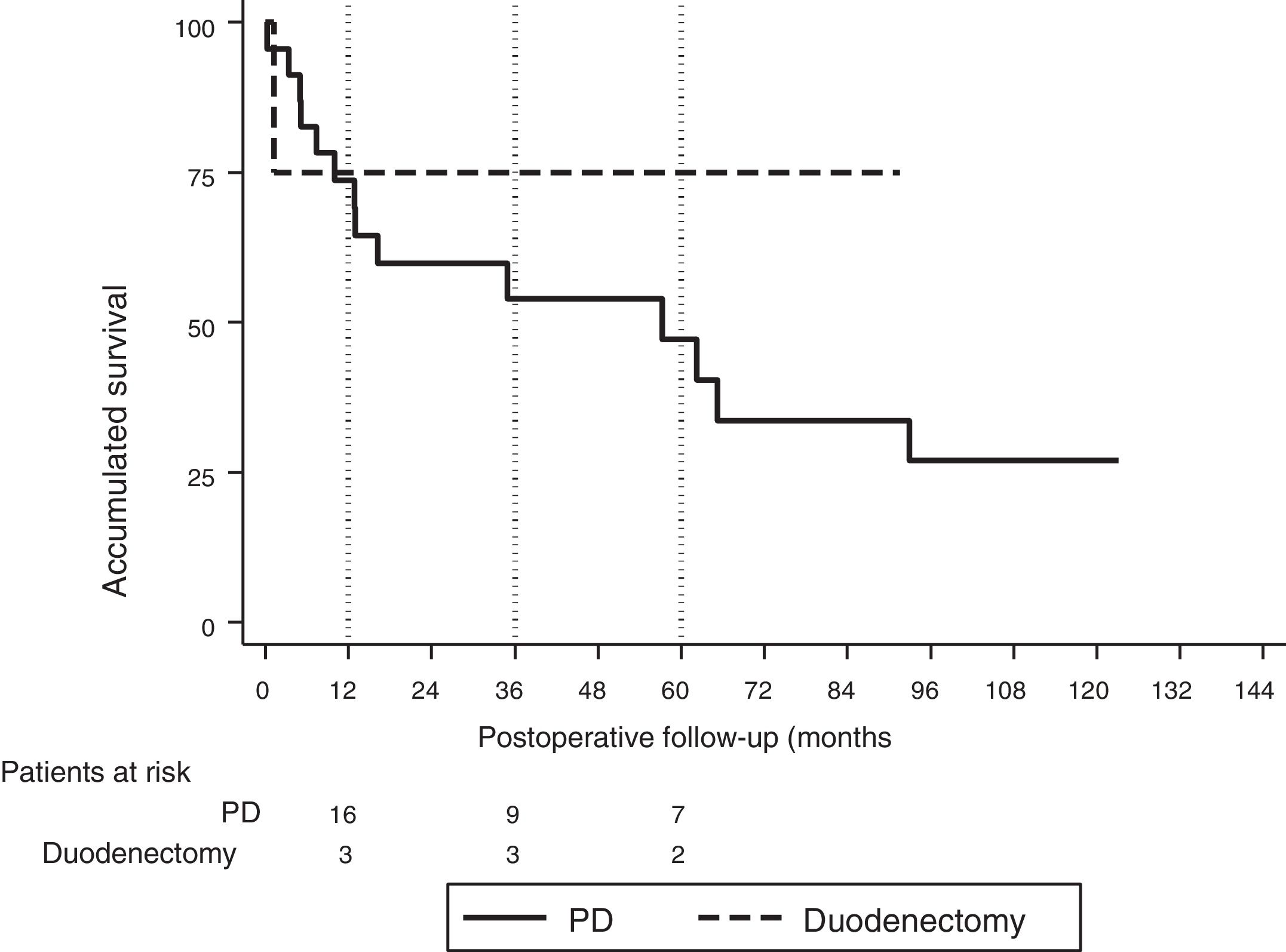
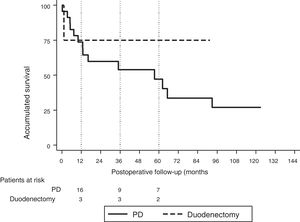
The median follow-up was 23 (9–69.7) months. During this period, 15 (55%) patients died (14 PD and 1 DD) and 12 (44%) patients were alive (9 PD and 3 DD). The overall 1, 3 and 5-year survival rates in PD were 74, 54 and 47%, respectively. In DD, the 1, 3 and 5-years rates were all 75%. No statistically significant differences were found between the two techniques (Fig. 2).
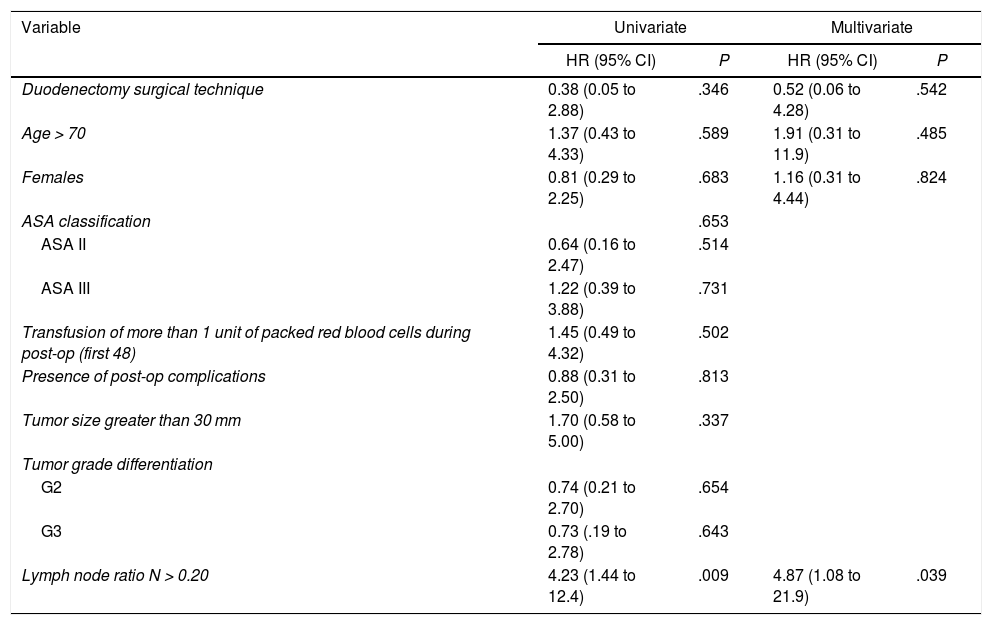
The median overall survival was 62.2 (25.2–99.1) months. Out of all the variables analyzed in the univariate study, only the lymph node ratio greater than 0.20 was a risk factor for long-term mortality (P = .009), a result that was confirmed by the multivariate analysis (Table 5).
Study of Risk Factors for Long-term Mortality After Surgery for Duodenal Adenocarcinoma (Hospital Universitari de Bellvitge, 1990–2017).
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Duodenectomy surgical technique | 0.38 (0.05 to 2.88) | .346 | 0.52 (0.06 to 4.28) | .542 |
| Age > 70 | 1.37 (0.43 to 4.33) | .589 | 1.91 (0.31 to 11.9) | .485 |
| Females | 0.81 (0.29 to 2.25) | .683 | 1.16 (0.31 to 4.44) | .824 |
| ASA classification | .653 | |||
| ASA II | 0.64 (0.16 to 2.47) | .514 | ||
| ASA III | 1.22 (0.39 to 3.88) | .731 | ||
| Transfusion of more than 1 unit of packed red blood cells during post-op (first 48) | 1.45 (0.49 to 4.32) | .502 | ||
| Presence of post-op complications | 0.88 (0.31 to 2.50) | .813 | ||
| Tumor size greater than 30 mm | 1.70 (0.58 to 5.00) | .337 | ||
| Tumor grade differentiation | ||||
| G2 | 0.74 (0.21 to 2.70) | .654 | ||
| G3 | 0.73 (.19 to 2.78) | .643 | ||
| Lymph node ratio N > 0.20 | 4.23 (1.44 to 12.4) | .009 | 4.87 (1.08 to 21.9) | .039 |
ASA: American Society of Anesthesiologists; CI: confidence interval; HR: hazard ratio.
Out of the total series, 13 patients (48%) had recurrence, 10 of whom died and 3 are still alive. All these patients had been treated by PD. The 1, 3 and 5-year disease-free actuarial survival rates were 71, 55 and 49%, respectively. The median disease-free survival was 49 (0–133) months (Table 4). None of the variables analyzed was a risk factor for long-term recurrence in the univariate or multivariate study.
DiscussionThere are few publications in the literature analyzing the surgical results of DA.16 The choice of surgical technique (PD vs. DD) is based on the location of the tumor and the presence of pancreatic infiltration. Classically, the technique of choice for DA was PD6 because it was considered the only method that could offer acceptable cancer outcomes. Subsequently, publications emerged with comparable results in terms of survival for DD and PD.4,17,18 Even so, there is still controversy about which oncological approach is the most appropriate, and there are authors who currently defend PD as the best treatment.11 In our experience, morbidity after resection for DA is 65% in the PD group and 75% in the DD group, with no statistically significant differences, which are results similar to other authors.4,19 However, several groups19,20 defend DD as a technique with similar postoperative morbidity. The postoperative mortality in our series was 7%, which is similar to publications by others.1,4,19,21–23 Like our results, other authors have compared postoperative mortality between PD and DD, finding no statistically significant differences.4,19 Postoperative mortality in our DD group due to adenocarcinoma was high (1 out of 4 patients); however, the cause of death of the patient in this group was not related to the surgical technique. When we reviewed our experience in DD for different indications, we have found a lower mortality rate (4%).24,25
Several prognostic factors have been proven to influence DA survival. Some of them refer to patient age,23,26 sex,27 tumor differentiation10,26 and tumor stage.26 In the pathological study, the variables related with the prognosis of DA, so far, are the involvement of the resection margin,1,4,10,18,21 number of resected lymphadenopathies, involvement of resected lymphadenopathies and the lymph node ratio.4,7,21,22,26,28,29 In our series, the involvement of the resection margin was zero in both study groups, with an actuarial 5-year survival after resection of 52%. Several authors have demonstrated that survival is affected if there is involvement of the resection margins. For instance, Sohn et al.10 published a 5-year survival rate of 58% in the case of R0 and 0% in the case of R1. Similar values are described by Poultsides et al.21 with a 5-year survival of 55% in R0 and 0% in R1.
The importance of lymphadenectomy is also well documented in the literature, despite there being no consensus on the number of nodes to resect.4,7,21,22,26 The AJCC recommends resecting a minimum of 6 nodes in DA surgery, although there are authors who believe that this number should be greater for better staging.3,21,28 Sarela et al.23 found statistically significant differences in 5-year survival between pN0 and pN+ patients when the number of resected nodes was equal to or greater than 15. In contrast, if the number of resected nodes was less than 15, the 5-year survival results were similar between both groups. In our study, we registered a greater number of nodes after PD compared to DD (22 vs. 12.5; P = .016), although this did not imply better survival. Other authors demonstrated similar results to our experience. Sakamoto et al.11 published a series with an average of 26 resected lymph nodes in the PD group vs. 14 nodes in the DD group (P = .001), with no differences in survival between the two groups. Along the same lines, Cloyd et al.26 showed results of a lymphadenectomy with a greater number of lymph nodes in the PD compared to DD (11 ± 8.9 vs. 6.8 ± 7.8; P < .0001), with no changes in long-term survival between the two groups. Several authors have shown that the lymph node ratio (affected nodes/resected nodes) is the most appropriate prognostic predictor.21,26,28,29 In our series, the lymph node ratio was the only variable that was proven to be a prognostic factor for long-term mortality. Finally, we have not observed differences in survival when comparing the 2 surgical techniques (PD and DD).4,11,26 In our series, the overall survival after resection for DA was 62.2 months and 52% after 5. These results are slightly higher than other published reports, with a survival between 30–45 months4,19,22,27 and a 5-year survival of 23%–48%.1,4,19,21,22
Being a rare entity, the number of patients in our study is low, despite a prolonged period of study. Therefore, it is difficult to draw conclusions, which is one of the limitations of this study. However, after analyzing our experience and that of other authors, we believe that both surgical options can be considered, depending on the tumor location and the degree of pancreatic involvement.
ConclusionSurgical resection of DA involves high postoperative morbidity, even though it is able to prolong survival. Depending on the location and in the absence of pancreatic invasion, segmental DD with free margins is an alternative to PD.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: López-Domínguez J, Busquets J, Secanella L, Peláez N, Serrano T, Fabregat J. Adenocarcinoma duodenal: resultados del tratamiento quirúrgico de una serie unicéntrica de 27 pacientes Cir Esp. 2019;97:523–530.