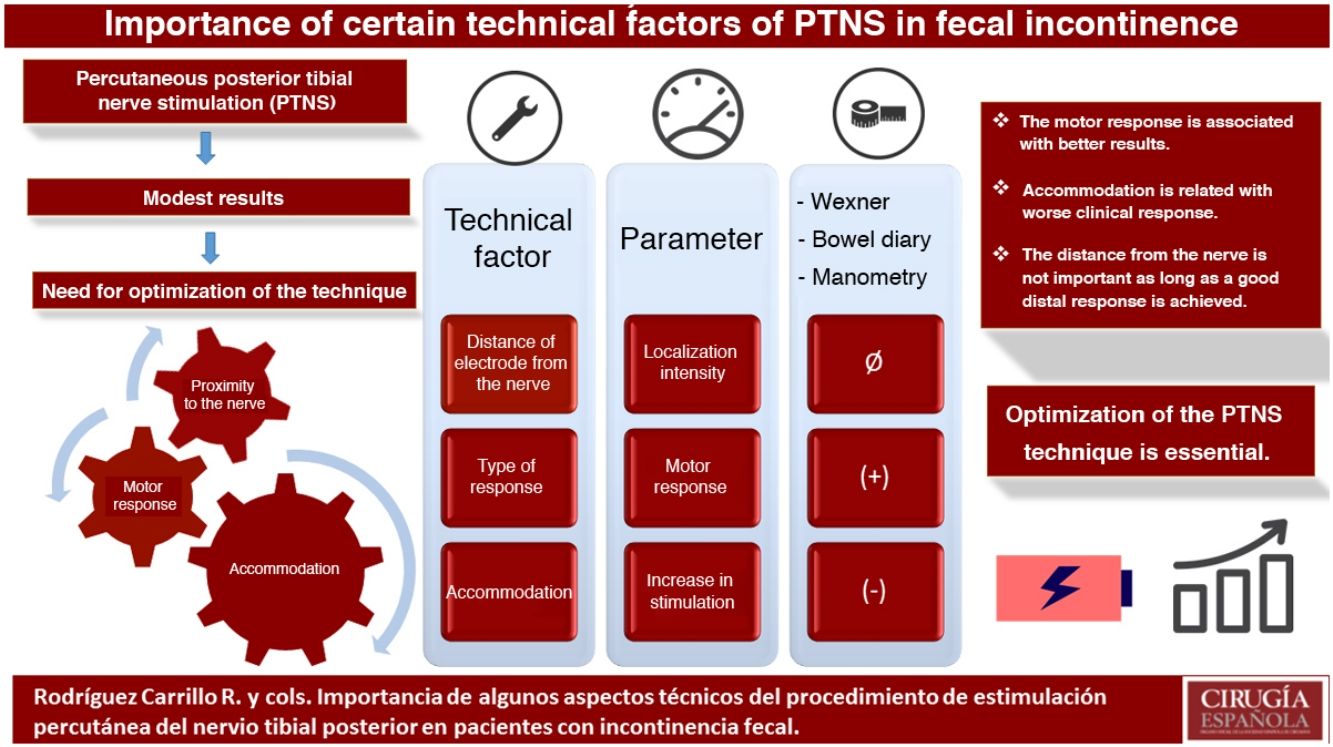
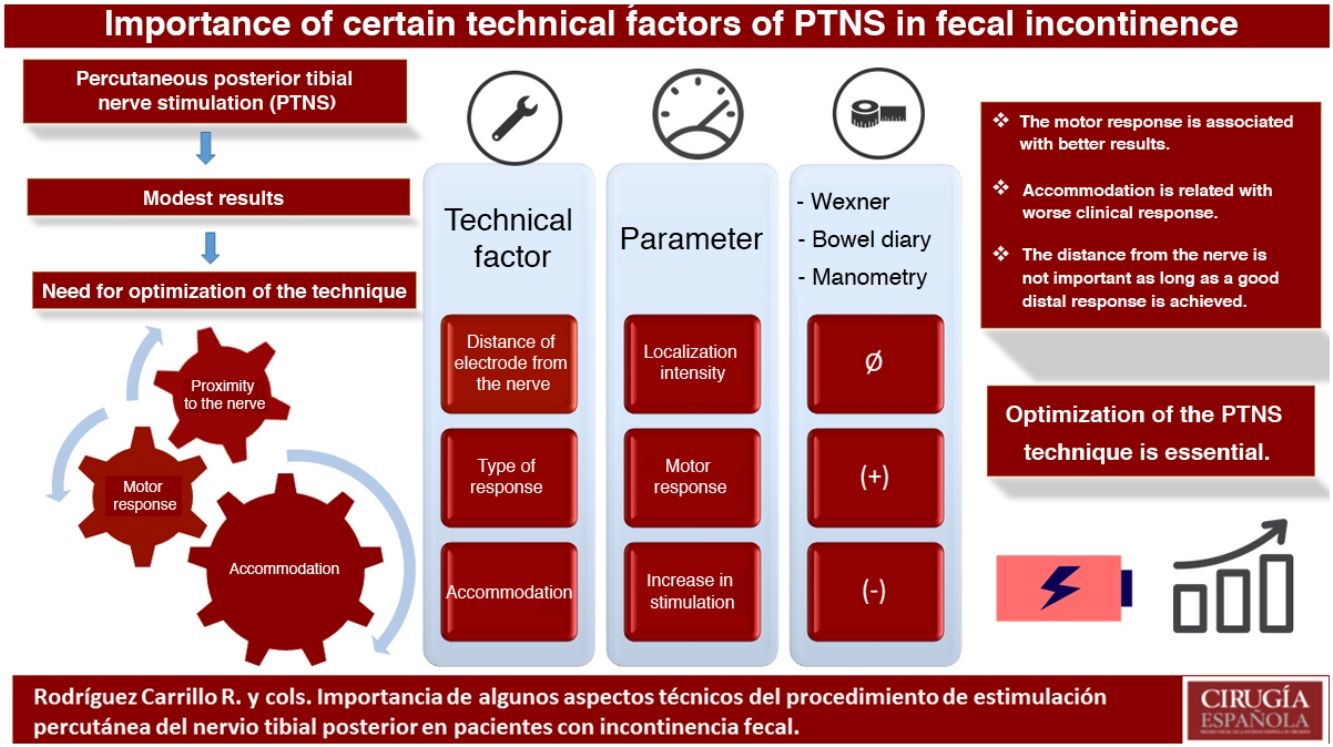
The results of percutaneous posterior tibial nerve stimulation (PTNS) in the treatment of fecal incontinence (IF) are modest. The aim of the study is to assess the relationship of some technical aspects with the clinical response: location of the nerve, distal response (motor or sensory) and accommodation.
MethodsProspective study of patients with FI undergoing PTNS therapy. The clinical response was assessed using the Wexner scale, defecation diary and anorectal manometry.
Results32 patients were studied. The intensity of localization (proximity to the nerve) was not correlated with clinical or manometric changes. Motor response was associated with a decrease on the Wexner scale [12.12 (±5.39) to 7.71 (±4.57) P < .005], the number of episodes of passive incontinence [8.78 (±9.64) to 4.11 (±7.11) P = .025], the total number of incontinence episodes [16.11 (±16.03) to 7.78 (±11.34) P = .009] and the number of days with fecal soiling [6.89 (±5.53) to 2.56 (±4.13) P = .002] and with an increase in the length of the manometric anal canal at rest [4.55 (±0.596) to 4.95 (±0.213) P = .004]. The increase in stimulation (accommodation) was inversely correlated with the decrease in the Wexner scale (r = −0.677 P < .005) and the number of days with soiling (r = −0.650 P = .022).
ConclusionsThe motor response during PTNS seems to be related to a better clinical response. The accommodation phenomenon could be associated with worse results. The proximity of the electrode to the nerve does not seem to be important as long as a good distal response is achieved.
Los resultados de la estimulación percutánea del nervio tibial posterior (PTNS) en el tratamiento de la incontinencia fecal (IF) parecen discretos. El objetivo del estudio es valorar la relación de algunos aspectos técnicos con la respuesta clínica: localización del nervio, respuesta distal (motora o sensitiva) y acomodación.
MétodosEstudio prospectivo de pacientes con IF sometidos a terapia de PTNS. La repuesta clínica se valoró mediante la escala de Wexner, diario defecatorio y manometría anorrectal.
ResultadosSe estudiaron 32 pacientes. La intensidad de localización (cercanía al nervio) no se correlacionó con cambios clínicos ni manométricos. La respuesta motora se relacionó con un descenso en la escala de Wexner [12,12 (±5,39) a 7,71 (±4,57) p < 0,005], el número de episodios de incontinencia pasiva [8,78 (±9,64) a 4,11 (±7,11) p = 0,025], el número total de episodios de incontinencia [16,11 (±16,03) a 7,78 (±11,34) p = 0,009] y el número de días con ensuciamiento fecal [6,89 (±5,53) a 2,56 (±4,13) p = 0,002] y con un aumento de la longitud del conducto anal manométrico en reposo [4,55 (±0,596) a 4,95 (±0,213) p = 0,004]. El incremento de estimulación (acomodación) se correlacionó de forma inversa con la disminución en la escala de Wexner (r = −0,677 p < 0,005) y el número de días con ensuciamiento (r = −0,650 p = 0,022).
ConclusionesLa respuesta motora durante la PTNS parece relacionarse con una mejor respuesta clínica. El fenómeno de acomodación podría asociarse con peores resultados. La cercanía del electrodo al nervio no parece tener trascendencia, siempre que se consiga una buena respuesta distal.
Percutaneous tibial nerve stimulation (PTNS) is a therapeutic procedure used for the treatment of fecal incontinence (FI). The results from published series have been modest, showing improved symptoms and quality of life1. Its main advantage over other therapies is its low invasiveness and low cost2. Despite this, some randomized studies have questioned its true effectiveness3. Two recent systematic reviews have determined that PTNS is related with a decrease in FI episodes, while showing no effects on the severity scales or manometric data4,5.
From a physiological standpoint, neuromodulation methods are believed to act mainly through the generation of action potentials in somatic afferent neurons that project to the medulla, where they can modulate visceral reflex circuits. In addition, this depolarization can reach higher pathways to the brain and produce effects at this level6.
During electrostimulation of a peripheral nerve, depolarization occurs both proximally and distally. The nerve fibers of mixed nerves, such as the tibialis, are more or less susceptible to depolarization depending on the level of intensity and frequency of stimulation. Mild intensity stimulation initially activates large-diameter sensory afferent fibers and motor efferent fibers through a spinal reflex called the H reflex. Increased intensity of the stimulus will cause direct motor efferent stimulation7,8. The duration of the physiological effect of these types of response can vary depending on the characteristics of the stimulated nerve in terms of its fibril composition9. In addition, during stimulation of the posterior tibial nerve, somatosensory evoked potentials can be registered at the cortical level through stimulation above the motor threshold10.
Therefore, technical optimization of PTNS seems important in order to improve the degree of stimulation of the corresponding nerve pathways and possibly to be able to improve clinical results. There are no studies in the literature that have evaluated the effects of variations in stimulation parameters in PTNS. Thus, the objective of this study is to evaluate the impact of some technical aspects of the PTNS on the clinical response of patients with FI: the distal response during stimulation (sensory or motor), the proximity of the electrode to the tibial nerve, and the phenomenon of accommodation during therapy.
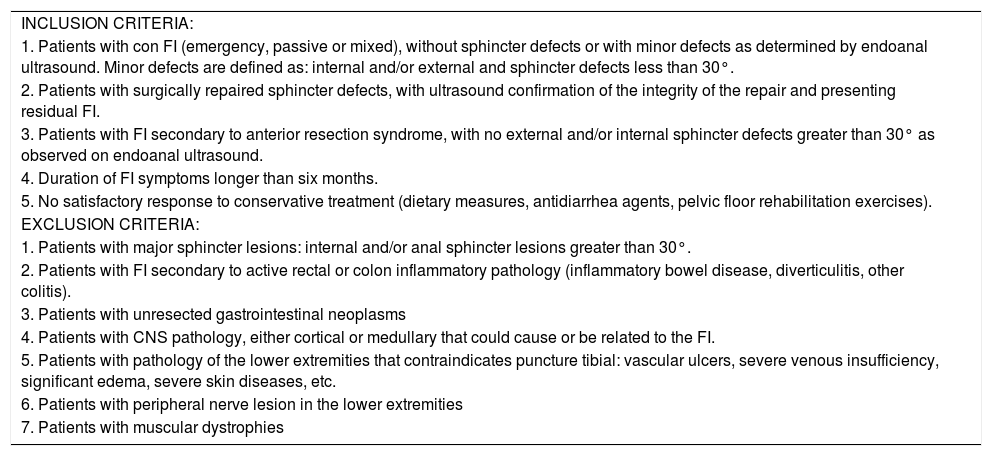
MethodsWe conducted a prospective study of patients with FI who had been treated with PTNS between May 2014 and November 2015. The inclusion and exclusion criteria are described in Table 1. The study was approved by the Clinical Research Ethics Committee of our hospital.
Study selection criteria.
| INCLUSION CRITERIA: |
| 1. Patients with con FI (emergency, passive or mixed), without sphincter defects or with minor defects as determined by endoanal ultrasound. Minor defects are defined as: internal and/or external and sphincter defects less than 30°. |
| 2. Patients with surgically repaired sphincter defects, with ultrasound confirmation of the integrity of the repair and presenting residual FI. |
| 3. Patients with FI secondary to anterior resection syndrome, with no external and/or internal sphincter defects greater than 30° as observed on endoanal ultrasound. |
| 4. Duration of FI symptoms longer than six months. |
| 5. No satisfactory response to conservative treatment (dietary measures, antidiarrhea agents, pelvic floor rehabilitation exercises). |
| EXCLUSION CRITERIA: |
| 1. Patients with major sphincter lesions: internal and/or anal sphincter lesions greater than 30°. |
| 2. Patients with FI secondary to active rectal or colon inflammatory pathology (inflammatory bowel disease, diverticulitis, other colitis). |
| 3. Patients with unresected gastrointestinal neoplasms |
| 4. Patients with CNS pathology, either cortical or medullary that could cause or be related to the FI. |
| 5. Patients with pathology of the lower extremities that contraindicates puncture tibial: vascular ulcers, severe venous insufficiency, significant edema, severe skin diseases, etc. |
| 6. Patients with peripheral nerve lesion in the lower extremities |
| 7. Patients with muscular dystrophies |
All patients who met the selection criteria were included in the study after obtaining informed consent. Treatment consisted of a weekly 30-minute session of unilateral PTNS for 8 weeks. The technical description of the procedure was described in a recent publication by our group11.
Stimulation parametersThe following parameters were recorded in each stimulation session:
Localization intensity: Minimum level of intensity that led to a sensory response (paresthesia in the heel, sole or toes). This is related to the distance from the electrode to the nerve (the lower the intensity, the shorter the distance).
Starting intensity: Intensity level at the start of each session that corresponded with the intensity that caused the maximum sensory and/or motor response tolerable by the patient.
Ending intensity: The intensity level recorded just before the end of the session. This is the level of intensity reached after the necessary increments during the session to maintain the sensory and/or motor response due to the physiological accommodation of the patient to the stimulation.
Distal response: Response observed during stimulation, which can be sensory (paresthesia in the sole, heel, or toes) and/or motor (flexion of one or more toes). For the statistical analysis, the most frequent response obtained in the total number of sessions was considered an independent variable.
Increase for localization: Difference between the starting intensity and the localization intensity.
Increase in stimulation: Difference between the intensity at the end and at the beginning. This is related to the phenomenon of physiological accommodation of the patient, making it necessary to increase the intensity in order to maintain the response to stimulation.
Clinical assessmentAll patients were evaluated for FI severity using the Wexner scale12 prior to treatment and 2–4 weeks after its completion.
In addition, a 21-day defecation diary was used prior to treatment and until the last session, which the patient sent by mail after completion. The patient was urged to complete the diary within 21 days after the end of therapy. The following variables were studied: number of stools, number of fecal urgency episodes, number of episodes of urgent fecal leakage, number of passive fecal leakage episodes, total number of incontinence episodes, number of days with soiling, and number of days when an incontinence pad was used.
Functional assessmentAn anorectal manometry study was performed prior to the start of treatment and 2–4 weeks after its completion.
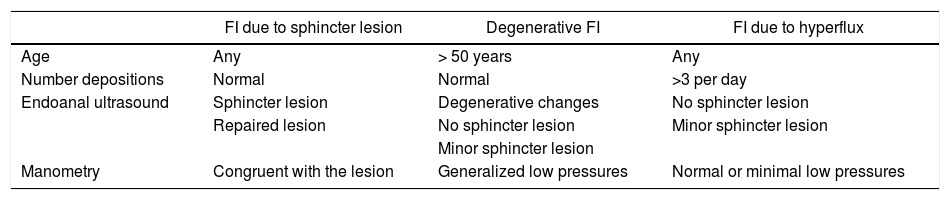
FI classificationThe type of FI in each patient was characterized from in terms of morphology (active or non-active sphincter lesion), semiology (urgency, passive or mixed) and etiology (due to repaired/unrepaired sphincter lesion, degenerative or hyperflux). Criteria for the etiological classification are described in Table 2.
Criteria for the etiological classification.
| FI due to sphincter lesion | Degenerative FI | FI due to hyperflux | |
|---|---|---|---|
| Age | Any | > 50 years | Any |
| Number depositions | Normal | Normal | >3 per day |
| Endoanal ultrasound | Sphincter lesion | Degenerative changes | No sphincter lesion |
| Repaired lesion | No sphincter lesion | Minor sphincter lesion | |
| Minor sphincter lesion | |||
| Manometry | Congruent with the lesion | Generalized low pressures | Normal or minimal low pressures |
For the data analysis, we used the IBM SPSS Statistics 23.0 package for Windows.
The measures of central tendency and dispersion of the different variables are expressed in the text as arithmetic mean and standard deviation (SD). The Student’s t test was used for paired data to compare variables before and after treatment, and Pearson’s correlation coefficient was used to measure the degree of relationship of different quantitative variables.
ResultsPatient characteristicsWe evaluated 32 patients (28 women, 87,5%), with a median age (IQR) of 63 (±19) years. Five patients were diabetic (16.6%). In 9 patients (28.1%), sphincter defects were observed on endoanal ultrasound. The distribution by groups according to the morphological, etiological or semiological type of FI is shown in Tables 3 and 4.
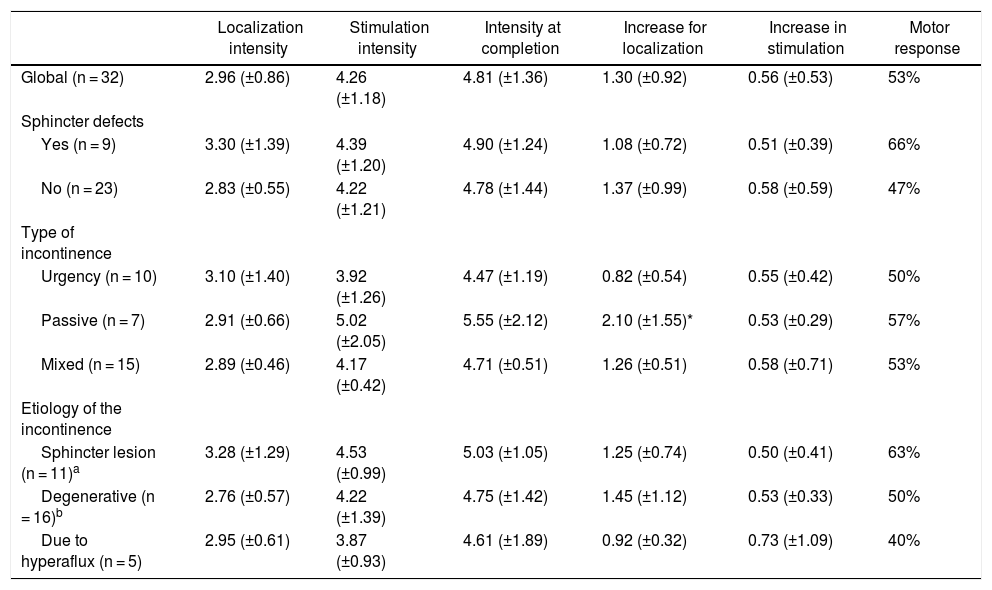
Mean levels (±SD) of stimulation and % of motor response in the global series and in the different subgroups.
| Localization intensity | Stimulation intensity | Intensity at completion | Increase for localization | Increase in stimulation | Motor response | |
|---|---|---|---|---|---|---|
| Global (n = 32) | 2.96 (±0.86) | 4.26 (±1.18) | 4.81 (±1.36) | 1.30 (±0.92) | 0.56 (±0.53) | 53% |
| Sphincter defects | ||||||
| Yes (n = 9) | 3.30 (±1.39) | 4.39 (±1.20) | 4.90 (±1.24) | 1.08 (±0.72) | 0.51 (±0.39) | 66% |
| No (n = 23) | 2.83 (±0.55) | 4.22 (±1.21) | 4.78 (±1.44) | 1.37 (±0.99) | 0.58 (±0.59) | 47% |
| Type of incontinence | ||||||
| Urgency (n = 10) | 3.10 (±1.40) | 3.92 (±1.26) | 4.47 (±1.19) | 0.82 (±0.54) | 0.55 (±0.42) | 50% |
| Passive (n = 7) | 2.91 (±0.66) | 5.02 (±2.05) | 5.55 (±2.12) | 2.10 (±1.55)* | 0.53 (±0.29) | 57% |
| Mixed (n = 15) | 2.89 (±0.46) | 4.17 (±0.42) | 4.71 (±0.51) | 1.26 (±0.51) | 0.58 (±0.71) | 53% |
| Etiology of the incontinence | ||||||
| Sphincter lesion (n = 11)a | 3.28 (±1.29) | 4.53 (±0.99) | 5.03 (±1.05) | 1.25 (±0.74) | 0.50 (±0.41) | 63% |
| Degenerative (n = 16)b | 2.76 (±0.57) | 4.22 (±1.39) | 4.75 (±1.42) | 1.45 (±1.12) | 0.53 (±0.33) | 50% |
| Due to hyperaflux (n = 5) | 2.95 (±0.61) | 3.87 (±0.93) | 4.61 (±1.89) | 0.92 (±0.32) | 0.73 (±1.09) | 40% |
(Equivalencies for levels of intensity: 1 = 0.15 mA; 2 = 0.5 mA; 3 = 1 mA; 4 = 1.5 mA; 5 = 2 mA; 6 = 2.5 mA; 7 = 3 mA; 8 = 3.5 mA; 9 = 4 mA; 10 = 4.5 mA; 11 = 5 mA; 12 = 5.5 mA; 13 = 6 mA; 14 = 6.5 mA; 15 = 7 mA; 16 = 7.5 mA; 17 = 8 mA; 18 = 8.5 mA; 19 = 9 mA).
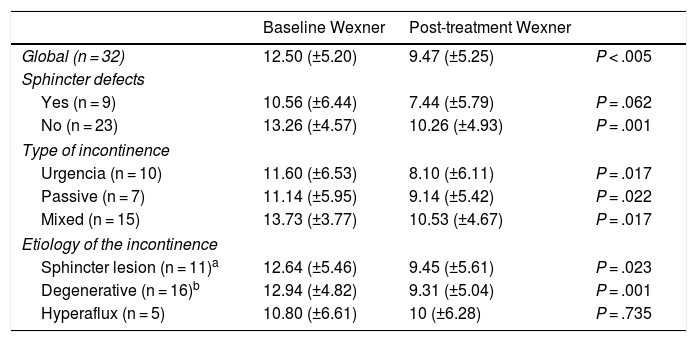
Mean scores (±SD) on the Wexner scale, showing baseline and after the completion of treatment in the global series and by groups.
| Baseline Wexner | Post-treatment Wexner | ||
|---|---|---|---|
| Global (n = 32) | 12.50 (±5.20) | 9.47 (±5.25) | P < .005 |
| Sphincter defects | |||
| Yes (n = 9) | 10.56 (±6.44) | 7.44 (±5.79) | P = .062 |
| No (n = 23) | 13.26 (±4.57) | 10.26 (±4.93) | P = .001 |
| Type of incontinence | |||
| Urgencia (n = 10) | 11.60 (±6.53) | 8.10 (±6.11) | P = .017 |
| Passive (n = 7) | 11.14 (±5.95) | 9.14 (±5.42) | P = .022 |
| Mixed (n = 15) | 13.73 (±3.77) | 10.53 (±4.67) | P = .017 |
| Etiology of the incontinence | |||
| Sphincter lesion (n = 11)a | 12.64 (±5.46) | 9.45 (±5.61) | P = .023 |
| Degenerative (n = 16)b | 12.94 (±4.82) | 9.31 (±5.04) | P = .001 |
| Hyperaflux (n = 5) | 10.80 (±6.61) | 10 (±6.28) | P = .735 |
Apart from medical treatment, four patients had previously received other specific treatments for FI: anal sphincteroplasty (3 patients), and sacral root neuromodulation (one patient, performed prior to PTNS therapy in our unit).
Stimulation characteristicsThe different mean levels of intensity and the type of response recorded are shown in Table 3. No significant differences were found in any group, except in the increase in intensity for localization, which was significantly greater in the passive incontinence group.
Wexner scaleAll patients were evaluated using the Wexner scale before and after treatment. In the global series, the Wexner scale score dropped significantly after the end of treatment from 12.60 (±5.20) to 9.47 (±5.25) (P < .005). This significant decrease was maintained in all morphological, etiological and semiological groups, except in the hyperflux incontinence group and in the group with sphincter injury (Table 4).
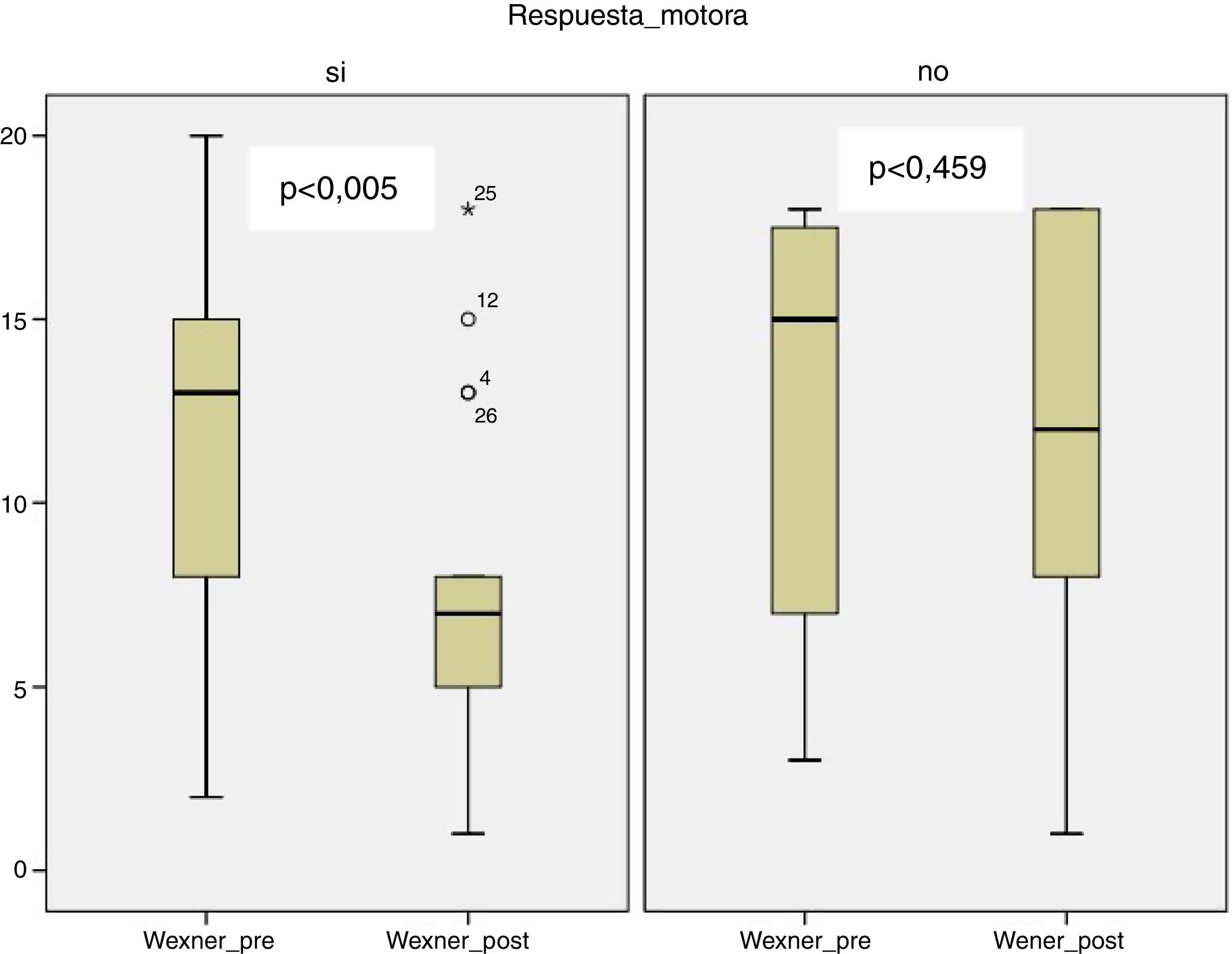
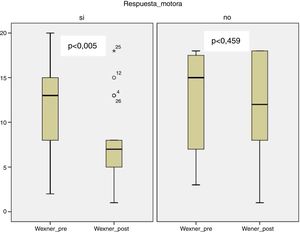
In the group with motor response, there was a significant improvement in the Wexner scale after treatment (12.12 [±5.39] to 7.71 [±4.57]; P < .005), unlike the group with sensory response (12.27 [±5.83] to 11.55 [±6.23] P = .459) (Fig. 1).
The decrease of more than 50% on the Wexner scale was achieved in 21.9% of the global series, specifically 35.6% of the group with motor response and 9.1% of the group with sensory response (P = .191).
A significant and inverse correlation was observed between the mean intensity at completion (r = −0.383, P = .044) and the mean increase in stimulation (r = −0.677, P < .005) with the decrease in the Wexner score after treatment.
Defecation diaryOnly 14 patients filled out the defecation diary correctly (in time and manner).
In the global series, a significant decrease was only found in the number of days of soiling after therapy (8.43 [±6.56] to 5.07 [±7.49]; P = .009).
In the group of patients with motor response, a significant reduction was reached in the number of passive incontinence episodes (8.78 [±9.64] to 4.11 [±7.11]; P = .025), the total number of incontinence episodes (16.11 [±16.03] to 7.78 [±11.34]; P = .009), and the number of days with fecal soiling (6.89 [±5.53] to 2.56 [±4.13]; P = .002). This significant reduction was not observed in the group with a sensory response.
A significant and inverse correlation was found between the mean increase in stimulation and the decrease in the number of days with fecal soiling in the bowel diary (r = −0.650; P = .022).
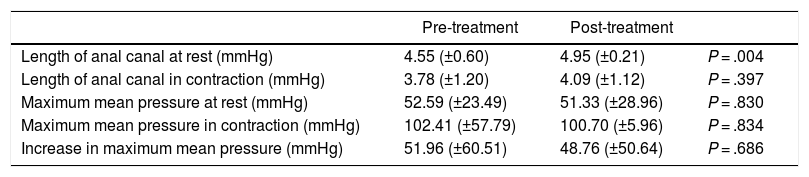
Anorectal manometryThe pre- and post-treatment manometric study was completed in 27 patients. The functional results are described in Table 5.
Mean manometry values (±SD), baseline and after the completion of treatment.
| Pre-treatment | Post-treatment | ||
|---|---|---|---|
| Length of anal canal at rest (mmHg) | 4.55 (±0.60) | 4.95 (±0.21) | P = .004 |
| Length of anal canal in contraction (mmHg) | 3.78 (±1.20) | 4.09 (±1.12) | P = .397 |
| Maximum mean pressure at rest (mmHg) | 52.59 (±23.49) | 51.33 (±28.96) | P = .830 |
| Maximum mean pressure in contraction (mmHg) | 102.41 (±57.79) | 100.70 (±5.96) | P = .834 |
| Increase in maximum mean pressure (mmHg) | 51.96 (±60.51) | 48.76 (±50.64) | P = .686 |
In the global series, a significant increase in the manometric length of the anal canal at rest was observed after therapy (4.55 [±0.596] to 4.95 [±0.213]; P = .004), with no evidence of other changes in the remaining variables. This effect was maintained in the group with a motor response (4.44 [±0.63] to 4.94 [±0.25]; P = .006), disappearing in the group with a sensory response.
No significant correlations were found between any of the electrical intensity parameters and variations in any of the main manometric variables.
DiscussionClinical improvement after the application of PTNS in patients with FI has been demonstrated in some publications, although this does not seem to be very important from a quantitative point of view. Reductions in the Wexner scale after therapy range between 3 and 4 points in most series13–25. However, these small changes can be very well accepted by patients, as has been shown in studies with structured qualitative assessments26. In our global series, the scores decreased from 12.60 to 9.47, with significant differences. This decrease was observed in all subgroups, except in the group with incontinence due to hyperflux (including three cases of anterior resection syndrome) and the group with sphincter injury, although in the latter the differences approached statistical significance (Table 4). There are studies that have demonstrated the effectiveness of the therapy in patients with anal sphincter injuries16,27 and in patients with anterior resection syndrome28.
The intensities used in the series (Table 3) were homogeneous in the different groups, except for the need for a higher initial intensity in the group of patients with passive incontinence. The explanation for this phenomenon could be a lower nerve excitability in this group of patients. The mean intensity at the start of stimulation was 4.26 (±1.19) (equivalent to 1.5 mA). This intensity was lower than that published by other authors, such as De la Portilla: 9 (±2.7) (equivalent to 4 mA)13. This is probably due to the fact that our group tries to place the electrode as close as possible to the tibial nerve. However, according to our data, the proximity of the electrode (localization intensity) has no clinical implications as long as an adequate response is achieved. This datum is consistent with the fact that the results of transcutaneous tibial stimulation are equivalent to percutaneous stimulation5, taking into account that the stimulation is done further away from the nerve fibers.
Although efferent distal stimulation lacks ‘direct’ clinical implications when stimulating the lower limb during PTNS (contrary to what occurs with other procedures, such as sacral neuromodulation), it could have some important connotations. First, in the stimulation of a peripheral nerve, efferent depolarization is achieved at above-threshold intensities of spinal reflex excitability7,8. Therefore, when there is a motor response, it is possible that the triggering of these reflexes is being ensured. In addition, the detection of somatosensory evoked potentials in the brain is achieved with stimuli that exceed the motor threshold10. In our study, in the group of patients in whom a distal motor response was achieved, better clinical response was observed after therapy, including a significant decrease in Wexner scale scores, the number of passive incontinence episodes, and the total number of episodes of incontinence and the number of days with fecal soiling. From a functional point of view, an increase was observed in the length of the anal canal at rest in this group. In a recent meta-analysis, it was not possible to demonstrate manometric changes after PTNS, but global series with motor and sensory responses were evaluated5.
Like other groups13,19, our group increased the level of intensity each time the sensation decreased as a result of accommodation. Other groups29,30 systematically raise the amplitude 1 mA after 15 min of treatment. It is uncertain whether subsensory stimulation in PTNS can be effective or not. In sacral neuromodulation studies, the effectiveness of this subsensory stimulation has been demonstrated31; however, it is a continuous stimulation and, on the other hand, afferent and efferent. In our study, although we tried to ensure that the stimulation was suprasensory throughout the session, the need for increased stimulation due to greater accommodation correlated with worse results on the Wexner scale and in the bowel diary. The meaning of this fact is difficult to specify, but it could be related to a worse nerve transmission capacity in patients who accommodate more.
This study has several limitations. The foremost is the loss of patients, especially in the completion of the defecation diaries. This is probably due to the very rigorous parameters for properly completing them in a timely manner (just after the end of the treatment to assess the immediate effects) and the fact that it is a 21-day questionnaire, so correct completion was more difficult. Second, the intrinsic heterogeneity of patients with FI is a constant fact in all series. In our study, clear inclusion criteria have been used to minimize this factor. Finally, we do not provide medium or long-term results, since the objective of the study was to assess the impact of technical parameters on immediate response, avoiding the possible effect of loss of response over time.
In conclusion, there are some technical aspects of the PTNS procedure that could be of clinical relevance. First of all, the closeness of the electrode to the tibial nerve does not seem to be associated with a greater effect, as long as a good distal response is achieved, even at the expense of increasing the intensity of the stimulation. In addition, achieving a motor response seems to be related with better results. Finally, patients who develop greater accommodation during therapy may respond more poorly to therapy.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Rodríguez Carrillo R, Ruiz Carmona MD, Alós Company R, Frangi Caregnato A, Alarcón Iranzo M, Solana Bueno A, et al. Importancia de algunos aspectos técnicos del procedimiento de estimulación percutánea del nervio tibial posterior en pacientes con incontinencia fecal. Cir Esp. 2021;99:585–592.