Wistar and Sprague Dawley (SD) rats are generally used as models for the cholesterol metabolism experiments. They are acceptable to high fat diet-induced disorders with individual variations, including dyslipidemia and abnormal cardiac pathology.
ObjectiveTo compare the effects of high fat diet in inducing dyslipidemia and cardiac pathological alterations between Wistar and SD rats.
MethodsWe compared the differences in plasma cholesterol levels and cardiac pathological alterations between Wistar and SD rats of standard diet (3.90 kcal/g) and high fat diet (5.40 kcal/g) after 4 weeks.
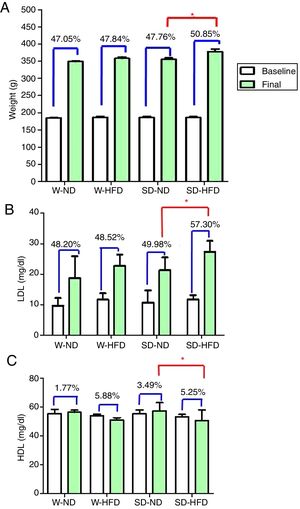
ResultsSD rats fed with high fat diet showed significantly enhanced LDL concentration and the decreased HDL concentration when compared to Wistar rats. Additionally, SD rats showed cardiac pathological alterations such as infiltration of mononuclear cells referring to inflammatory response and high amounts of perivascular fat playing a key role in the impairment of vascular functions.
ConclusionsOur results indicate that SD rats may be the more suitable model for dyslipidemia and alteration of cardiac pathology induced by high fat diet.
Las ratas Wistar y Sprague Dawley (SD) se usan generalmente como modelos para los experimentos del metabolismo del colesterol. Son aceptables para los trastornos inducidos por la dieta alta en grasas con variaciones individuales, incluida la dislipidemia y la patología cardiaca anormal.
ObjetivoComparar los efectos de la dieta alta en grasas en la inducción de dislipidemia y alteraciones patológicas cardiacas entre ratas Wistar y SD.
MétodosComparamos las diferencias en los niveles de colesterol en plasma y las alteraciones patológicas cardkacas entre ratas Wistar y SD de dieta estándar (3,90 kcal/g) y dieta alta en grasas (5,40 kcal/g) después de 4 semanas.
ResultadosLas ratas SD alimentadas con una dieta alta en grasas mostraron una concentración significativamente mejorada de LDL y una concentración disminuida de HDL en comparación con las ratas Wistar. Además, las ratas SD mostraron alteraciones patológicas cardiacas, como la infiltración de células mononucleares que se refieren a la respuesta inflamatoria y las altas cantidades de grasa perivascular que juegan un papel clave en el deterioro de las funciones vasculares.
ConclusionesNuestros resultados indican que las ratas SD pueden ser el modelo más adecuado para la dislipidemia y la alteración de la patología cardiaca inducida por una dieta alta en grasas.
Research animals are considered as valuable tools for understanding pathophysiology and in developing therapeutic interventions in diseases for many decades. Rat is one of the most widely used research animals for studying physiology, pharmacology, and metabolism in basic medical research.1,2 The benefits of using rat models in biomedical researches are the following: rats are easy to manage in terms of dietary feeding and controlling environmental factors including temperature, humidity, and lighting. As the result, nearly all environmental variations do not differ much from human studies. In addition, rats generally have shorter life span than human. The changes and adaptation can be observed throughout their life cycle or even across several generations. The blood and organs can be also collected for profound experiment and bio-molecular investigations. Moreover, anatomical basis and physiological functions in rats, especially cardiac pathology, are similar to humans as well.3
Dyslipidemia can be present in rats with abnormal cholesterol metabolism. Inducing dyslipidemia in rats is often through a high fat diet using variety of fats such as lard, coconut, soybean, or palm oil, etc. It may be because prolonged ingestion of saturated fats is directly related to dyslipidemia and atherosclerosis.4
In experiment associated with cholesterol metabolism, Wistar and Sprague Dawley (SD) rats are vulnerable to diet-induced obesity and dyslipidemia with other variations including the alterations of cardiac pathology.5 Previous studies reported that feeding diet containing as little as 42% fat in both Wistar and SD rats can change peripheral cholesterol levels in various circumstances.6–9 Nevertheless, there are influencing variations such as age, feeding duration, and fat composition in diet used that may also be affect the experimental outcomes.6 Therefore, the present study aimed to compare the effects of high fat diet in inducing dyslipidemia and cardiac pathological alterations between Wistar and SD rats.
Materials and methodsChemicals and reagentsCommercial kits for lipid profiles (BioSystems S.A, Barcelona, Spain), formalin buffer solution, neutral buffered, 10% (Sigma–Aldrich, St Louis, MO, United States), paraffin–polyisobutylene mixture (Leica Biosystems, Teban Gardens Crescen, Singapore), hematoxylin – eosin solution (Sigma–Aldrich, St Louis, MO, United States).
AnimalsFive-week-old male Wistar rat and Sprague Dawley rats weighing 180–190g were purchased from the National Laboratory Animal Center at Salaya Campus, Mahidol University. Two rats were housed in a plastic cage, opening on the top and covered with a rod lid in a room at 55–65% ambient humidity maintained and temperature controlled at 20–24°C under the 12-h light-dark cycle. The rats were raised with free access to drinking water and standard diet for 1 week before being assigned to the variable dietary groups. All procedures were conducted and approved by the Animal Care Ethical Committee of Laboratory Animal Science Center, Faculty of Tropical Medicine, Mahidol University (Approval No. FTM-ACUC 010/2018). FTM-ACUC memberships include doctor of veterinary medicine, researcher experienced in research involving animals and nonscientific background public member. The ethical standards and procedures were according to the Guide for the Care and Use of Laboratory Animals.10
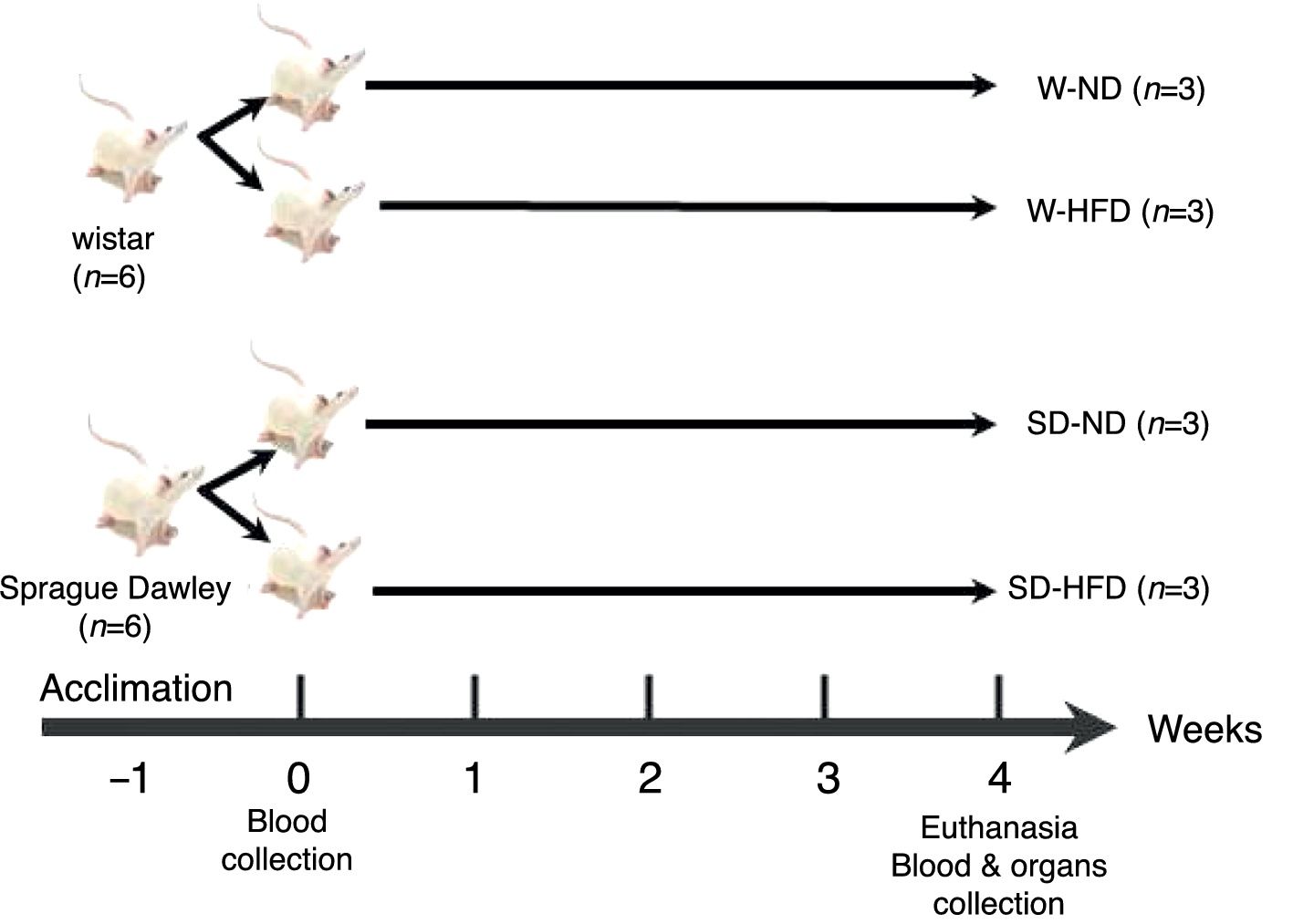
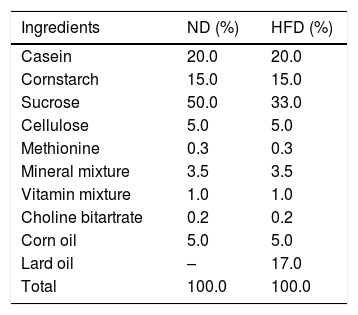
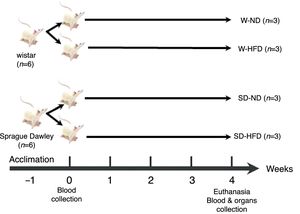
Experimental designOur experimental design consisted of 3 rats per experimental group. The strategic rationale for sample size calculation of experimental groups come from equation of n1= [(Z1−α/2+Z1−β)2 (σ12+[σ22/r])]/δ2 which n1=sample size, σ=standard deviation, r=n2/n1, δ=difference between two group of means. The sample size was calculated according to total cholesterol5 using power (1−β)=0.9 and type I error (α)=0.01. After acclimation for 1 week, the 12 rats, 6 Wistar rats and 6 Sprague Dawley rats (SD) were divided into 4 groups (n=3) for 2 different diets; standard diet (ND) or high fat diet (HFD) for 4 weeks according to the experimental design (Fig. 1). The standard diet and HFD were prepared according to the American Institute of Nutrition (AIN) 76A formula and D12492 formula with some modifications. The standard diet contained 11.5% fat, 20.8% protein, 67.7% carbohydrate and 3.90kcal/g, and HFD consisted of 58.3% fat, 20.2% protein and 21.5% carbohydrate and 5.40kcal/g. The composition of diets is shown in Table 1. At the end of the experiment, each rat was overnight fasted and euthanized with carbon dioxide. The heart and the adipose tissue were collected, weighed and kept for further analysis.
The experimental design of twelve rats. Both Wistar and Sprague Dawley (SD) rats (6 per each) were randomly divided into 4 groups and fed in selective diets for 4 weeks. W-ND; Wistar rats fed standard diet, W-HFD; Wistar rats fed a high fat diet, SD-ND; Sprague Dawley rats fed standard diet, SD-HFD; Sprague Dawley rats fed a high fat diet.
The composition of rat diets.
| Ingredients | ND (%) | HFD (%) |
|---|---|---|
| Casein | 20.0 | 20.0 |
| Cornstarch | 15.0 | 15.0 |
| Sucrose | 50.0 | 33.0 |
| Cellulose | 5.0 | 5.0 |
| Methionine | 0.3 | 0.3 |
| Mineral mixture | 3.5 | 3.5 |
| Vitamin mixture | 1.0 | 1.0 |
| Choline bitartrate | 0.2 | 0.2 |
| Corn oil | 5.0 | 5.0 |
| Lard oil | – | 17.0 |
| Total | 100.0 | 100.0 |
ND: standard diet, HFD: a high fat diet.
Body weight, food, and water consumption were measured and recorded. The amount of food consumed was calculated for calorie intake. Weight gain and weight of the visceral fat were determined. Serum total cholesterol (TC), high density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and triglyceride (TG) from lateral tail vein were measured by commercial kits for lipid profiles.
Histopathological analysisAfter euthanasia, the heart was harvested, trimmed of fat, and weighed. The middle cross-section of the heart was fixed on a filter paper in a 10% formalin buffer solution, and the fixed tissues were dehydrated and subsequently embedded in a paraffin–polyisobutylene mixture. Then, they were sectioned (4μm) on slides and stained with hematoxylin and eosin (H&E).11 To facilitate a histopathological evaluation of ventricular cardiac tissue, a light microscope (Zeiss Axio Observer A1, Germany) with an attached camera (Canon, USA) and the ZoomBrowser EX computer-based image analysis software (Canon, USA) were used. The morphological changes were classified based on vascular morphological changes which were assessed by two independent researchers.
Statistical analysisNumerical data were analyzed using SPSS version 18.0 statistics software (Chicago, IL, USA). Data are represented as means±standard deviations. Group differences were analyzed using the analysis of variance (ANOVA), followed by a post hoc Tukey's test as appropriate. The p value of less than 0.05 was acceptable for a statistically significant difference. Graphs were performed using GraphPad Prism 5 statistical software (GraphPad Software Inc., La Jolla, CA, USA).
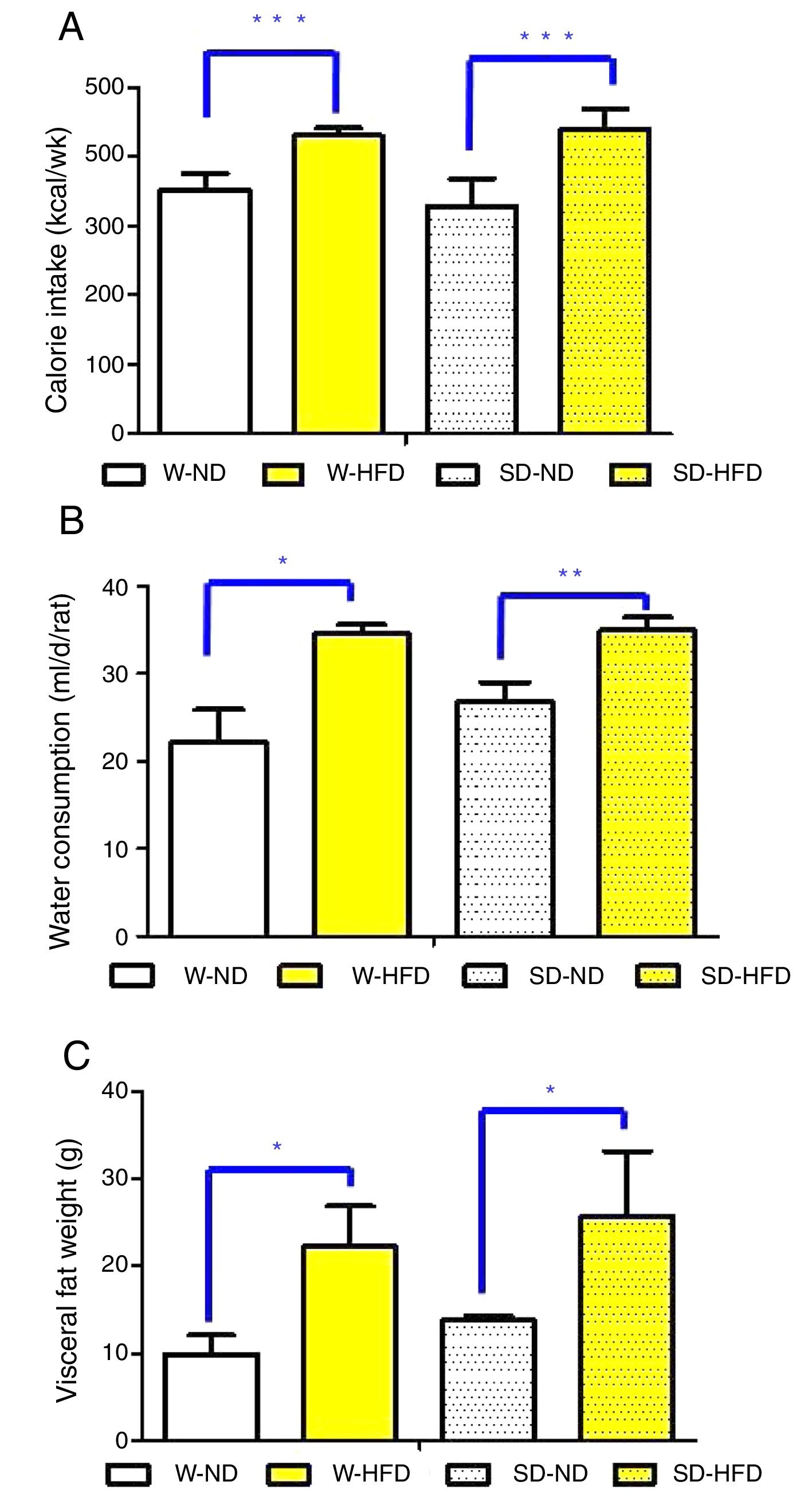
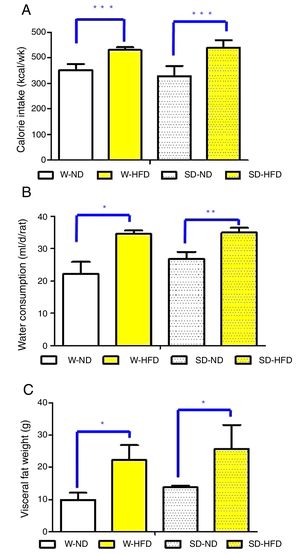
ResultsFood and water consumption, calorie intake, body weight and visceral fat weightAll animals survived and had no abnormal symptoms or behavioral changes throughout the study period. After feeding different types of diets for 4 weeks, food and water consumption in Wistar and Sprague Dawley (SD) rats were thoroughly observed. Although significant difference in food consumption was not found, there was significantly higher energy intake and water consumption in rats fed with HFD compared to those fed with ND (Fig. 2A and B).
The comparison of calorie intake (A), water consumption (B) and visceral fat weight (C) in Wistar and Sprague Dawley rats fed either standard diet (ND) or high fat diet (HFD). Data are expressed as mean±standard deviations. W-ND; Wistar rats fed standard diet, W-HFD; Wistar rats fed a high fat diet, SD-ND; Sprague Dawley rats fed standard diet, SD-HFD; Sprague Dawley rats fed a high fat diet. *p<0.05, **p<0.01, ***p<0.001.
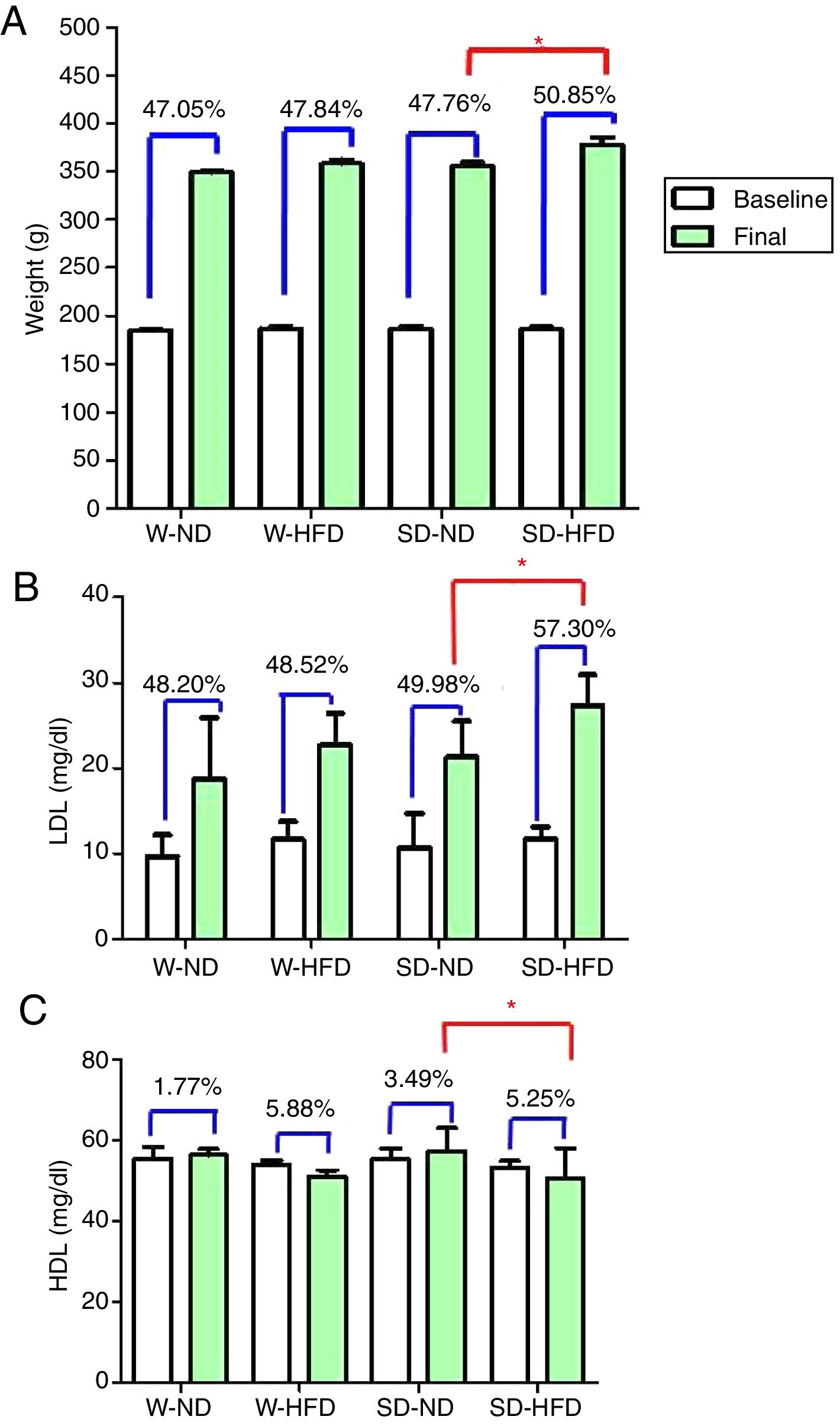
At baseline, Wistar and SD rats in the 4 divided groups had similar average initial body weight. High energy intake led to remarkably increased body weight, especially in SD-HFD group. The significant difference in body weight was found in SD-HFD group when compared with SD-ND group (Fig. 3A). Moreover, the significant differences of visceral fat weight were found in both Wistar and SD rats fed with standard diet when compared to those fed with high fat diet (Fig. 2C). No significant difference of visceral fat weight was found between Wistar and SD rats fed with a high fat diet. Additionally, the significant difference was not observed in weight of heart among different groups.
The body weight (A), LDL (B) and HDL (C) at baseline and at the end of the experiment. Data are expressed as mean±standard deviations. W-ND; Wistar rats fed standard diet, W-HFD; Wistar rats fed a high fat diet, SD-ND; Sprague Dawley rats fed standard diet, SD-HFD; Sprague Dawley rats fed a high fat diet. *p<0.05.
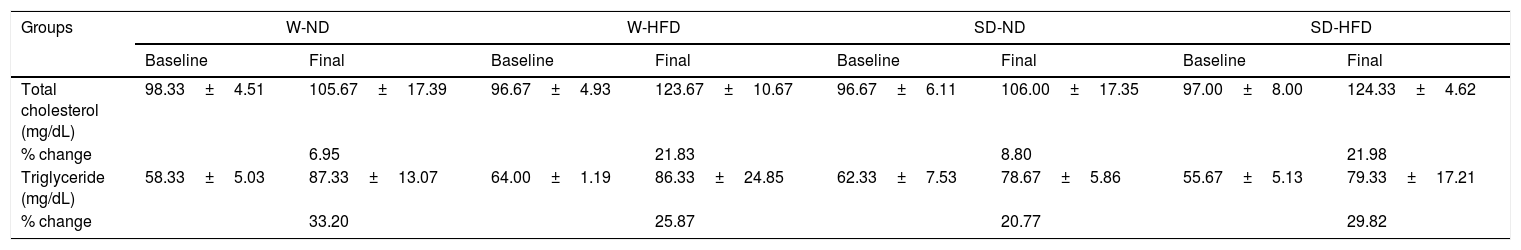
After feeding different kinds of diet in both strains of rats for 4 weeks, LDL levels increased from baseline. The significant increase of LDL was found in SD rats fed with HFD in comparison to rats in SD-ND groups (Fig. 3B). HDL levels changed from baseline (Fig. 3C). The significant decrease in HDL levels was found in SD rats fed with HFD in comparison to rats in SD-ND groups. Nevertheless, no significant changes in total cholesterol and triglyceride were observed (Table 2).
Changes of total cholesterol and triglyceride between at baseline and at the end of the experiment.
| Groups | W-ND | W-HFD | SD-ND | SD-HFD | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Baseline | Final | Baseline | Final | |
| Total cholesterol (mg/dL) | 98.33±4.51 | 105.67±17.39 | 96.67±4.93 | 123.67±10.67 | 96.67±6.11 | 106.00±17.35 | 97.00±8.00 | 124.33±4.62 |
| % change | 6.95 | 21.83 | 8.80 | 21.98 | ||||
| Triglyceride (mg/dL) | 58.33±5.03 | 87.33±13.07 | 64.00±1.19 | 86.33±24.85 | 62.33±7.53 | 78.67±5.86 | 55.67±5.13 | 79.33±17.21 |
| % change | 33.20 | 25.87 | 20.77 | 29.82 | ||||
Data are expressed as mean±SD, n=3. W-ND; Wistar rats fed standard diet, W-HFD; Wistar rats fed a high fat diet, SD-ND; Sprague Dawley rats fed standard diet, SD-HFD; Sprague Dawley rats fed a high fat diet. p<0.05 represent significant differences compared with rats fed standard diet in the same species. % change compared between final and baseline.
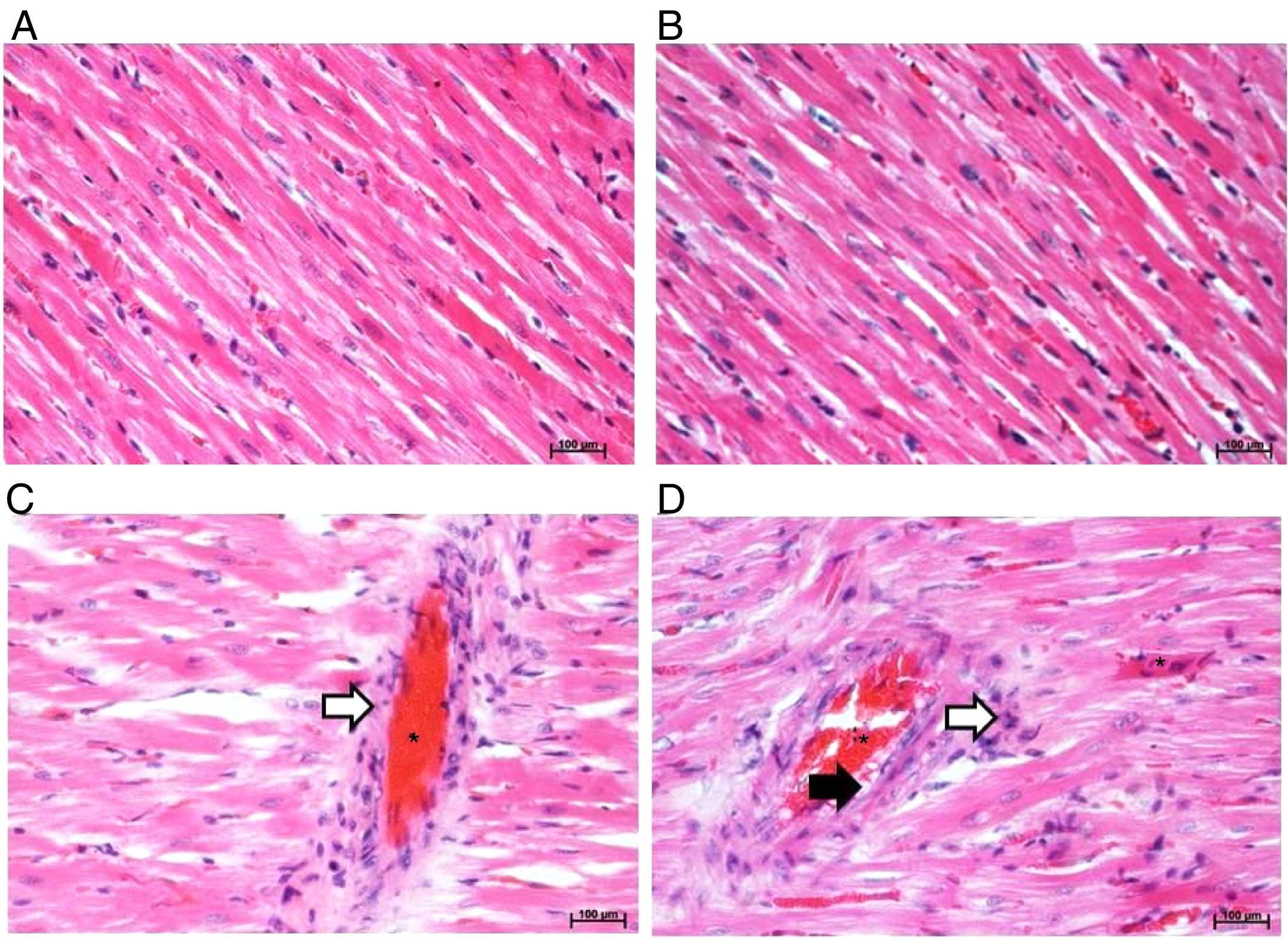
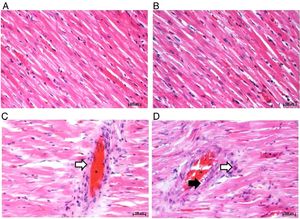
According to histopathology, microscopic images in Fig. 4A and B showed that the myocardial fibers of the rats fed with standard diet in both strains were found to be arranged in regular pattern with clear striations. There was no evidence of degeneration or necrosis. The nuclei appeared round or oval. In contrast, after feeding a high-fat diet for 4 weeks, the cardiac muscle structure of SD-HFD rats showed higher amounts of the mononuclear cell infiltration associated inflammation, dilated congestion of vascular related to myocyte damage, and enhancement of perivascular fat surrounding the blood vessel when compared with W-HFD rats (Fig. 4C and D).
Histopathology of H&E stained ventricular cardiac tissues (200x magnification). A: Wistar rat fed standard diet, B: SD rat fed standard diet, C: Wistar rat fed high fat diet and D: SD rat fed high fat diet. Asterisks represent dilated congestion of blood vessel. White arrows represent the accumulation of mononuclear cells associated with inflammation. Black arrow represents perivascular fat surrounding the blood vessel.
Obesity and overweight induced by high fat diet have been shown to be associated with cardiac pathology alterations.12,13 High fat diet can also have influences on increasing body weight, visceral fat, and lipoprotein metabolism. In the present study, the higher body and adipocyte weights were found in all groups of rats fed with high fat diet when compared to those fed with standard diet. Rats which received diet with excess energy may also synthesize and accumulate more adipose tissue as triacylglycerol14 than those which get fed with standard diet, resulting in higher visceral fat weight and the dyslipidemic effects associated with obesity and overweight.15
Our data also indicated that Sprague-Dawley (SD) rats showed more complicated effects from high fat diet consumption when compared to Wistar rat. Similarly to our study, other studies in SD rats showed that rats which were fed with 61% fat diet for 8 weeks,16 40–45% fat diet for 3–4 weeks17and 60–70% fat diet for 2 weeks18 could develop obesity and increase visceral fat mass. However, it was reported that Wistar rats could increase weight, body fat mass more pronouncedly than SD rats when fed with high fat diet for 17 weeks.5 This may be the reason that Wistar rats consumed higher amounts of food and energy than SD rats at that time.
Lard oil, a major fat of diet in this study, contains high levels of saturated fatty acid (SFA) and cholesterol which are the greatest adverse factors affecting LDL and HDL concentrations which are associated with cardiovascular diseases (CVD), such as arteriosclerosis, stroke, and myocardial infarction.7 Fatty acids regulate transcription factors, such as the peroxisome proliferator activated receptors (PPARs) and sterol regulatory element binding proteins (SREBPs) which are related to the synthesis and the reverse uptake of cholesterol, fatty acids, triglycerides, and phospholipids.19 Consequently, lard oil in this study contributed to obesity and dyslipidaemia in rats, especially in SD rats. Similar to the study of Jia and colleages,7 they studied in 3-week-male SD rats fed with high fat diet (4.39kcal/g, 47% calories from fat). After 4-week feeding diet, serum LDL concentrations of rats increased while HDL concentrations of rats significantly decreased. AbdulKadir and his team20 also showed that in 8-week-old male SD rats fed with high fat diet (4.14kcal/g and 40% fat) for 10 weeks, there was a significant increment of LDL anda significant decrement of HDL similarly to the results of Asrullah's study in SD rats fed with high fat diet for 28 days.21 Male Sprague Dawley rats fed with high-fat lard diet for 24 weeks also showed the significantly increased triglyceride (TG) levels.22
In recent study, cardiac pathological alterations were found in rats fed with high fat diet, especially in SD rats. The accumulation of mononuclear cells associated with inflammation and perivascular fat surrounding the blood vessel was evident. The appearance of mononuclear cell infiltration with extravasation of red blood cells in the heart tissue may indicate the inflammatory response.23 Stemmer suggested that obesity can induce the low-grade inflammation by releasing of inflammatory adipocytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) from enlarged adipose tissue. Due to these adipocytokines, they can adversely affect various non-adipose target tissues.24 In addition, perivascular fat surrounding the blood vessel was also observed in this study. Montani25 proposed that obesity could lead to lipid accumulation in tissues and even organs. Surrounding blood vessels, there are lipid deposits, called perivascular fat, which may affect vascular function at least through the secretion of proatherogenic cytokines. This can contribute to the increased vascular stiffness. Therefore, perivascular fat may be a key factor of the impairment of vascular functions and increasing cardiovascular diseases prevalence.
In this study, rats fed with high fat diet tended to consume higher amount of water. It is possibly because more water is required for the enzymatic reactions in lipid digestion and absorption. Water is essential for the integrity of the structure of protein molecule used for digestion, especially in enzymatic hydrolysis reactions.26 Lipase enzyme is one of the significant enzymes composed of water. It hydrolyzes triglycerides into diglycerides, monoglycerides, fatty acids, and glycerol.27,28 In addition, bile salt consists of approximate 95% of water. Its role is to emulsify dietary fats and facilitate their intestinal absorption which is the major route for elimination of cholesterol.29 For these reasons, rats fed with high fat diet had a tendency to drink more water than those fed standard diet.
ConclusionThe results of this study showed the enhancement of blood LDL, the low level of HDL concentrations, the infiltration of mononuclear cells, and the high amounts of perivascular fat in cardiac tissue in SD rats fed with high fat diet when compared to Wistar rats fed with the same diet. Therefore, SD rats may be the more suitable model for dyslipidemia induced by high fat diet.
Conflict of interestThe authors have no conflicts of interest to declare.
We are grateful to the Faculty of Tropical Medicine, Mahidol University and the National Research Council of Thailand for providing financial support to this study.