Ovarian cancer is ranked highest among gynecological cancers, followed by cervical and endometrial cancer. Most women are asymptomatic and are eventually diagnosed with late-stage disease. Numerous recent studies have proposed promising protein tumour biomarkers for diagnosis, prognosis, treatment and disease recurrence. Cancer antigen 125 (CA-125) and human epididymis protein 4 (HE4) are biomarkers routinely used for monitoring recurrence in ovarian cancer patients. They are of limited diagnostic value in early-stage cancer. Application of sensitive advanced proteomics techniques reveals that a combined biomarker panel is superior in specificity and sensitivity compared to a single biomarker. The major limitation in translating potential tumour biomarkers from the research setting to clinical practice is a lack of validation in large patient cohorts. This review provides an overview of current and potential biomarkers for ovarian, endometrial and cervical cancers. In conclusion, we propose validation studies for multiple biomarker panels of apolipoprotein A-I (ApoA-I)+CA-125+transthyretin and vascular cell adhesion molecule-1 (VCAM-1)+CA-125+carcinoembryonic antigen (CEA)+HE4 for early diagnosis of ovarian cancer. We also suggest combination panels of prognostic value consisting of CA-125+HE4 for endometrial cancer and squamous cell carcinoma antigen (SCC-Ag)+CEA for cervical cancer.
El cáncer de ovario ocupa el primer lugar entre los cánceres ginecológicos, seguido del cáncer de cuello uterino y del cáncer de endometrio. La mayoría de las mujeres son asintomáticas, por lo que finalmente se les diagnostica la enfermedad en una fase avanzada. En numerosos estudios recientes se han propuesto prometedores biomarcadores tumorales proteicos para el diagnóstico, el pronóstico, el tratamiento y la recidiva de la enfermedad. El antígeno de cáncer 125 (CA-125) y la proteína 4 del epidídimo humano son biomarcadores que se utilizan de forma habitual para controlar la recidiva en pacientes con cáncer de ovario. Tienen un valor diagnóstico limitado en las fases iniciales del cáncer. La aplicación de técnicas sensibles de proteómica avanzada ha revelado que un grupo combinado de biomarcadores es superior en especificidad y sensibilidad en comparación con un único biomarcador. La principal limitación a la hora de trasladar los posibles biomarcadores tumorales del ámbito de la investigación a la práctica clínica es la falta de validación en grandes cohortes de pacientes. Esta revisión ofrece una visión general de los biomarcadores actuales y potenciales para el cáncer de ovario, de endometrio y de cuello uterino. En conclusión, proponemos estudios de validación para varios grupos de biomarcadores de apolipoproteína A-I+CA-125+transtiretina y molécula de adhesión celular vascular 1+CA-125+antígeno carcinoembrionario+proteína 4 del epidídimo humano para el diagnóstico precoz del cáncer de ovario. También sugerimos grupos combinados de valor pronóstico, compuestos por CA-125+proteína 4 del epidídimo humano para el cáncer de endometrio y antígeno de carcinoma de células escamosas+ antígeno carcinoembrionario para el cáncer de cuello uterino.
Gynecological cancers form a heterogeneous cluster of tumors originating in the organs of the female reproductive system.1 Globally, ovarian cancer (OC) is ranked first among gynecological cancers with the highest incidence and morbidity rate, followed by cervical and endometrial cancer (EC).2 Whereas, vaginal and vulvar cancers are rare.1 Most of these malignancies cause either vague or no symptoms, leading to late-stage diagnosis and poor patient outcome. Cervical cancer is the only gynecological cancer that may be detected at an early stage through cervical exfoliative cytology (PAP smear) screening programs. Tumor biomarkers play a critical role in diagnostic, prognostic, predictive or therapeutic applications. The addition of biomarkers to currently available methods such as imaging will improve clinical decision-making and optimize patient management. An ideal biomarker is detectable at high levels in cancer patients compared to unaffected individuals and preferably measured using non- or minimally invasive clinical samples such as blood, urine, or saliva.3 Besides, biomarkers should be sensitive, specific, cost-effective, reliable, and have clinical utility. Molecular biomarkers in blood constitute various cellular elements such as circulating tumor cells, genetic (DNA and RNA) material, protein elements (protein and peptides), and metabolites.4 Of these, proteins remain a major substance of interest as they represent end products that control most of the cellular functions and biological processes.5 Proteins can be quantified efficiently, cost-effectively, and has high analytical sensitivity. Proteomics-based studies using contemporary technologies have generated many promising biomarkers for ovarian, endometrial and cervical cancers; however, no biomarkers were reported for vaginal and vulva cancers. This review provides a synopsis on the current and potential biomarkers in ovarian, endometrial and cervical cancers, and aims to encourage researchers to embark on necessary follow-up studies before translation into routine clinical practice.
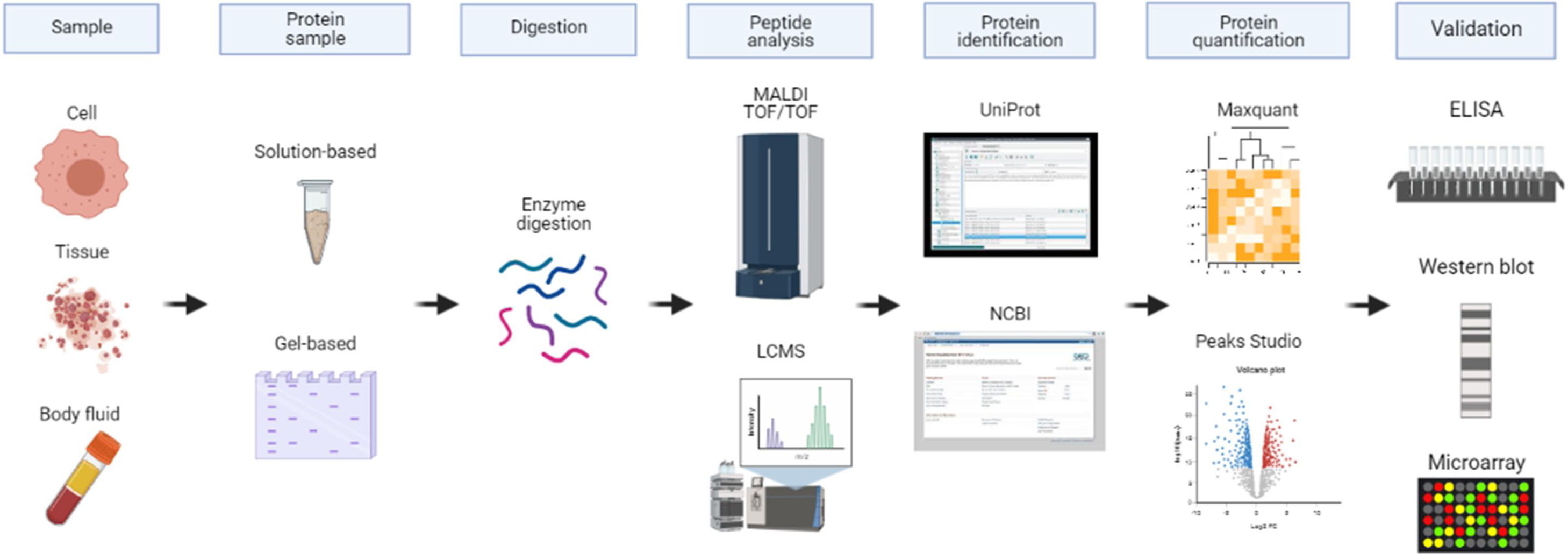
Proteomics approach in biomarker discoveryThe advancement in protein separation, identification, quantification and validation provides a better understanding of protein functions.6 The conventional proteomics method utilizes two-dimensional gel electrophoresis (2DE) which separates proteins according to their size and charge allowing visualization of large portions of proteomes.7 The introduction of fluorescent two-dimensional difference gel electrophoresis (2D-DIGE) led to enhanced protein separation and quantification. In general, gel-based methods are less sensitive in terms of qualitative and quantitative analyses. Complete characterization of proteomes can only be achieved using mass spectrometry techniques such as nanoflow liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS).8 Before MS analysis, the proteins are digested into peptides using a protease such as trypsin. The commonly used methods for protein digestion are in-gel digestion and in-solution digestion. Based on the MS/MS spectra of peptides generated, proteins are identified using database search engines such as UniProt/Swiss-Prot and/or NCBI sequence database to match the sequences.9,10 To accurately quantify proteins, label-free quantification (LFQ) or labelled-based approaches such as Multidimensional Protein Identification Technology (MudPIT), Isotope-Coded Affinity Tag (ICAT) and isobaric Tag for Relative and Absolute Quantitation (iTRAQ) are the methods of choice. The quantified proteins are analyzed with tools such as Maxquant and Peaks studio. Validation of the identified biomarkers is done using suitable assays including immunohistochemistry, Western Blot, and ELISA. Other immunoassay techniques for the detection of proteins in body fluids include Luminex bead assay, electrochemiluminescence immunoassay (ECLIA), and Simple Plex multi-analyte immunoassay. Fig. 1 illustrates the proteomics approach in biomarker discovery.
The proteomics approach in biomarker discovery. Various biological samples contain proteins including cells, tissues, and body fluids. The complex proteins are extracted from these samples. Next, they are stored in-solution or subjected to gel electrophoresis which separates the proteins. The extracted proteins are digested with suitable enzymes such as trypsin before using a mass spectrometry-based technique. Based on the peptides sequence results, the proteins are identified using database search engines for example UniProt or NCBI. For subsequent protein quantification, Maxquant or Peaks Studio bioinformatics tools are commonly used. The potential protein biomarkers are then validated using suitable assays such as immunoassay, Western Blot or protein microarray.
Although extensive research has been conducted to discover better biomarkers, CA125 has remained superior.11 CA125 is a membrane glycoprotein antigen expressed in Müllerian and coelomic epithelial tissue derivatives.12 It has proven value in the diagnosis and prognosis of ovarian cancer (OC). Approximately 80% of epithelial OC patients show elevated CA125 concentrations above 35U/ml. Reports have demonstrated elevated levels of CA125 in 50–60% of patients in clinical stage I, 80–90% in stage II, and more than 90% of patients in stages III to IV in epithelial ovarian cancer OC. CA125 can be diagnosed with 80% specificity and 92% sensitivity at late-stage OC,13 however, it has limited sensitivity and specificity for the diagnosis of early-stage cancer.14 Thus, CA125 is ideally used for cancer surveillance after a diagnosis of OC and hasn’t received approval by the US Food and Drug Administration (FDA) for preoperative use.15
Human epididymis 4 (HE4)In 2009, HE4 was approved by the FDA to monitor the progression and recurrence of epithelial OC in combination with CA125.16 HE4 is a protease inhibitor found in the epithelia of normal genital tissue.17 It is highly expressed by malignant epithelial ovarian cells and identified in the sera of individuals with OC.18 Analysis of serum samples demonstrated sensitivity ranging from 45.9 to 72.9% and 95% specificity. Interestingly, the sensitivity of serum HE4 increased to 76.5% when combined with CA125.19,20 Furthermore, HE4 has greater specificity than CA125 in the premenopausal age group as it is not elevated in benign gynecological conditions.21 The combination of serum proteins CA125, HE4, carcinoembryonic antigen (CEA), and vascular cell adhesion molecule-1 (VCAM-1) resulted in 86% sensitivity and 98% specificity.22
OsteopontinOsteopontin is an acidic, calcium-binding glycoprotein constituting the extracellular matrix.23 Under a stressed environment, osteopontin promotes ovarian cancer progression, cell survival, and metastasis, mediated through the activation and induction of hypoxia-inducible factor-1 (HIF-1α) expression.24 Elevated osteopontin expression is seen in borderline and invasive OC.25 For the diagnosis of OC, osteopontin sensitivity ranged from 80 to 85.4% at a specificity of 33.7% (stage I–IV) with a cut-off level of 252ng/ml.26 Furthermore, the sensitivity was reported to be elevated to 93.8% in combination with CA125.23 Using a multiplex ELISA panel comprising insulin-like growth factor, leptin, prolactin, macrophage inhibitory factor, CA125, and osteopontin, the sensitivity increased to 95.3% at a specificity of 99.4% for ovarian cancer detection.27 Though it is evident that a combination of osteopontin with other biomarkers shows promising results for the detection of ovarian cancer, the translation into clinical application has not been explored.
Transthyretin (TTR)Transthyretin is a transport protein in the blood with the ability to bind to thyroid hormones and retinol-binding protein.28 Alterations in serum TTR concentration are seen in several inflammatory conditions, thyroid disease, malnutrition, and other diseases.29 Cramer et al. reported transthyretin with 98% specificity and 47% sensitivity for both early and late-stage OC.30 A panel comprising transthyretin, CA125, ApoA1 and transferrin showed increased sensitivity of 76% with 98% specificity for early-stage OC.31 Also, improved sensitivity of 93.9% and specificity of 95% was reported by Kim et al., at all stages of OC using transthyretin, ApoA1 and CA125 panel.32
Cytokines (IL-6 and IL-8)Cytokines such as IL-6 and IL-8 are secreted by antigen-presenting cells (APCs), tumor cells, and tumor-derived fibroblasts in the ovary.33 The most common cytokine studied in ovarian cancer is IL-8.14 Concentrations of IL-8 and anti-IL-8 antibodies were found to be increased in stages I and II of OC with 65.5% sensitivity and 98% specificity.34 Whereas, IL-6 showed 86% specificity and 84.1% sensitivity for early-stage OC.35 A four-marker panel of CA125, HE4, E-cadherin, and IL-6 displayed 95.7% sensitivity and 84.2% specificity compared to individual markers, for early detection of serous ovarian cancer.36
KallikreinsHuman kallikreins (hKLK) encode the largest contiguous cluster of protease genes in the human genome. They have distinct expression patterns and pathological functions for angiogenesis, apoptosis, and metastasis in tumor cells.37 The kallikreins overexpressed in ovarian cancer at mRNA and protein levels comprise KLK4-8, KLK10-11, and KLK13-15.23 In OC, KLK11 was found to be elevated by 72% in serum at 90% specificity.38 When hK10 was combined with CA125, it achieved a greater sensitivity (73%) compared to hK10 (55%) or CA125 (60%) alone, at 90% specificity. Pre-operative high serum hK10 concentration was also noted to be an independent unfavorable prognostic factor for ovarian cancer.39
B7-H4B7-H4 negatively regulates T-cell immunity by the inhibition of T-cell cytokine production, proliferation, and cell cycle progression.40 Elevated serum B7-H4 protein demonstrated 65% sensitivity at 97% specificity when combined with CA125 at early-stage ovarian cancer.41 In another study conducted by Simon et al.,42 48% of patients at stage I, 55% of patients at stage II, and 67% of patients with late-stage ovarian cancer demonstrated high expression levels of serum B7-H4. B7-H4 may be useful as a potential biomarker for the diagnosis of early-stage cancer.
Apolipoprotein A1 (ApoA1)The main protein component in high-density lipoprotein with anti-atherogenic, antioxidant, and anti-inflammatory properties is apolipoprotein A1 (ApoA1).23 Studies related to ApoA1 and ovarian cancer are scarce though a decreased level of ApoA1 has been reported in ovarian cancer patients.43 A panel of ApoA1, CA125, and β-2-microglobulin (β2M) reached a sensitivity of up to 94% and specificity of 98% for detection of early-stage disease.44 In a study conducted by Clarke et al., a combination panel of ApoA1, a truncated form of transthyretin, connective tissue activating peptide III, and CA125 yielded a sensitivity of 84% at 98% specificity.45
Biomarkers for endometrial cancerTwo proteins widely evaluated in endometrial cancer are CA125 and HE4. Some studies have described the correlation of increased CA125 levels (>40U/mL) with higher grade, higher stage, increased depth of myometrial invasion, lymph node metastases, and presence of lymphovascular space involvement in endometrial cancer.46 At a cut-off level of 20U/mL of CA125, myometrial invasion to more than one-half of the myometrium could be diagnosed with a sensitivity of 69.0% and specificity of 74.1%.47 HE4 has a higher sensitivity than CA125 in the prognosis of endometrial cancer. HE4 was a better predictor of outer-half myometrial invasion than CA125, especially in patients with low-grade endometrioid tumors.48 HE4 provided a sensitivity ranging from 46 to 59.4% and specificity of 95 to 100% for endometrioid adenocarcinoma in all stages at a cut-off level of 70pmol/L.49,50
Biomarkers for cervical cancerSquamous cell carcinoma antigen (SCC-Ag)SCC-Ag is expressed in the normal squamous epithelium and can be used as a prognostic factor in cervical cancer. Elevated levels of serum SCC-Ag was found to be strongly correlated with poor prognosis and inferior progression-free survival.51 Also, a meta-analysis study revealed 1.1 – 40.0ng/mL cut-off levels in pre-treatment and 0.9–2.0ng/mL cut-off levels in post-treatment serum SCC-Ag in cervical cancer patients were associated with recurrence and mortality.52
Serum fragments of cytokeratin (CYFRA)CYFRA 21-1 is a serum fragment of cytokeratin 19, a subunit of cytokeratin expressed in normal epithelial cells and carcinomas of the cervix.53,54 Elevated levels of CYFRA 21-1 were observed in 42–63% of patients with cervical cancer.55 However, CYFRA 21-1 was less sensitive than SCC-Ag in the diagnosis of squamous cell carcinoma.56 CYFRA 21-1 level was related to prognostic factors such as stage, depth of stromal invasion, tumor size, and lymph node metastasis, while elevated pre-treatment levels indicated shorter disease-free survival in cervical cancer patients.56
Carcinoembryonic antigen (CEA)CEA has been widely studied for its role as a biomarker for early cancer diagnosis and as a prognostic indicator in many cancers, although its assessment in gynecological cancers is limited.53 The sensitivity of CEA for cervical cancer detection was 32% in squamous cell carcinoma and 38.5% in adenocarcinoma.57 In invasive squamous cell carcinoma, the percentage of patients with CEA values above 2.5ng/ml showed a gradual increase from 26 to 88% in stage I–IV, signifying the prognostic value of this marker.58
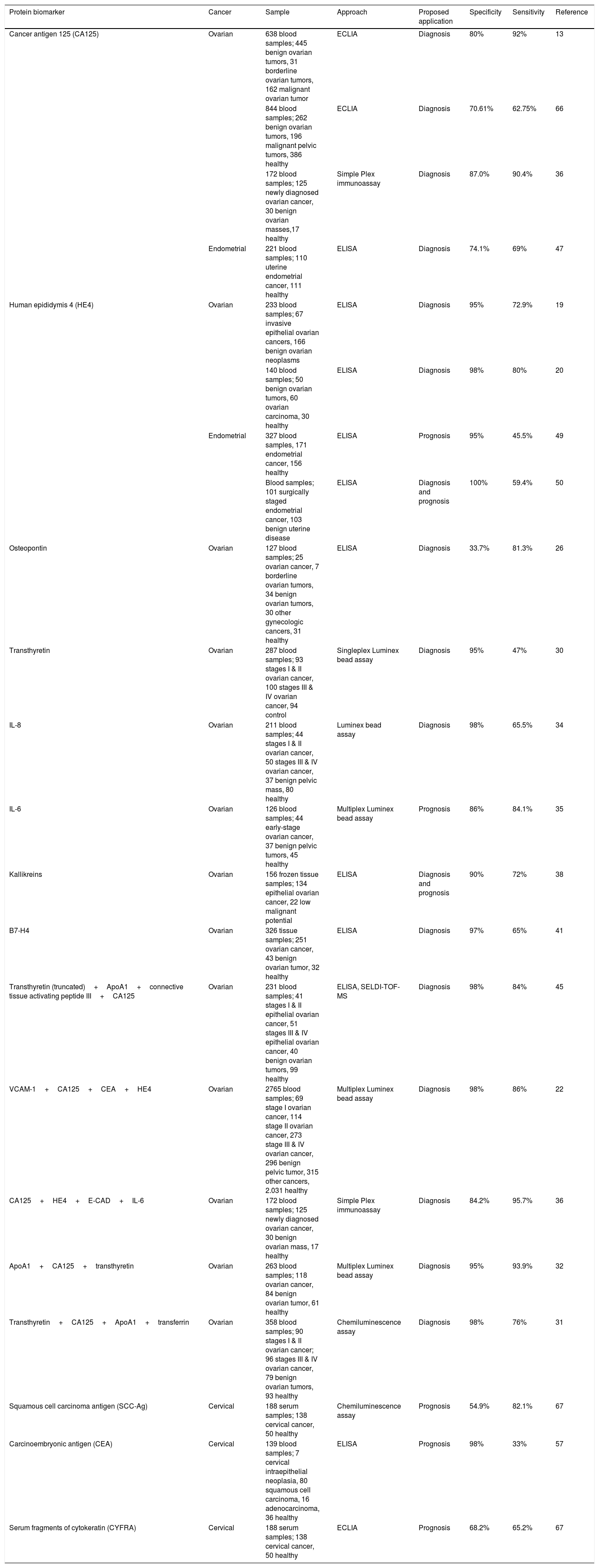
Table 1 summarises the most promising protein biomarkers as single or in combination for ovarian, endometrial and cervical cancers. These tumor biomarkers warrant further investigations and validation in large patient cohorts.
An overview of potential protein tumor biomarkers as a single or combination panel for ovarian, endometrial and cervical cancers.
| Protein biomarker | Cancer | Sample | Approach | Proposed application | Specificity | Sensitivity | Reference |
|---|---|---|---|---|---|---|---|
| Cancer antigen 125 (CA125) | Ovarian | 638 blood samples; 445 benign ovarian tumors, 31 borderline ovarian tumors, 162 malignant ovarian tumor | ECLIA | Diagnosis | 80% | 92% | 13 |
| 844 blood samples; 262 benign ovarian tumors, 196 malignant pelvic tumors, 386 healthy | ECLIA | Diagnosis | 70.61% | 62.75% | 66 | ||
| 172 blood samples; 125 newly diagnosed ovarian cancer, 30 benign ovarian masses,17 healthy | Simple Plex immunoassay | Diagnosis | 87.0% | 90.4% | 36 | ||
| Endometrial | 221 blood samples; 110 uterine endometrial cancer, 111 healthy | ELISA | Diagnosis | 74.1% | 69% | 47 | |
| Human epididymis 4 (HE4) | Ovarian | 233 blood samples; 67 invasive epithelial ovarian cancers, 166 benign ovarian neoplasms | ELISA | Diagnosis | 95% | 72.9% | 19 |
| 140 blood samples; 50 benign ovarian tumors, 60 ovarian carcinoma, 30 healthy | ELISA | Diagnosis | 98% | 80% | 20 | ||
| Endometrial | 327 blood samples, 171 endometrial cancer, 156 healthy | ELISA | Prognosis | 95% | 45.5% | 49 | |
| Blood samples; 101 surgically staged endometrial cancer, 103 benign uterine disease | ELISA | Diagnosis and prognosis | 100% | 59.4% | 50 | ||
| Osteopontin | Ovarian | 127 blood samples; 25 ovarian cancer, 7 borderline ovarian tumors, 34 benign ovarian tumors, 30 other gynecologic cancers, 31 healthy | ELISA | Diagnosis | 33.7% | 81.3% | 26 |
| Transthyretin | Ovarian | 287 blood samples; 93 stages I & II ovarian cancer, 100 stages III & IV ovarian cancer, 94 control | Singleplex Luminex bead assay | Diagnosis | 95% | 47% | 30 |
| IL-8 | Ovarian | 211 blood samples; 44 stages I & II ovarian cancer, 50 stages III & IV ovarian cancer, 37 benign pelvic mass, 80 healthy | Luminex bead assay | Diagnosis | 98% | 65.5% | 34 |
| IL-6 | Ovarian | 126 blood samples; 44 early-stage ovarian cancer, 37 benign pelvic tumors, 45 healthy | Multiplex Luminex bead assay | Prognosis | 86% | 84.1% | 35 |
| Kallikreins | Ovarian | 156 frozen tissue samples; 134 epithelial ovarian cancer, 22 low malignant potential | ELISA | Diagnosis and prognosis | 90% | 72% | 38 |
| B7-H4 | Ovarian | 326 tissue samples; 251 ovarian cancer, 43 benign ovarian tumor, 32 healthy | ELISA | Diagnosis | 97% | 65% | 41 |
| Transthyretin (truncated)+ApoA1+connective tissue activating peptide III+CA125 | Ovarian | 231 blood samples; 41 stages I & II epithelial ovarian cancer, 51 stages III & IV epithelial ovarian cancer, 40 benign ovarian tumors, 99 healthy | ELISA, SELDI-TOF-MS | Diagnosis | 98% | 84% | 45 |
| VCAM-1+CA125+CEA+HE4 | Ovarian | 2765 blood samples; 69 stage I ovarian cancer, 114 stage II ovarian cancer, 273 stage III & IV ovarian cancer, 296 benign pelvic tumor, 315 other cancers, 2.031 healthy | Multiplex Luminex bead assay | Diagnosis | 98% | 86% | 22 |
| CA125+HE4+E-CAD+IL-6 | Ovarian | 172 blood samples; 125 newly diagnosed ovarian cancer, 30 benign ovarian mass, 17 healthy | Simple Plex immunoassay | Diagnosis | 84.2% | 95.7% | 36 |
| ApoA1+CA125+transthyretin | Ovarian | 263 blood samples; 118 ovarian cancer, 84 benign ovarian tumor, 61 healthy | Multiplex Luminex bead assay | Diagnosis | 95% | 93.9% | 32 |
| Transthyretin+CA125+ApoA1+transferrin | Ovarian | 358 blood samples; 90 stages I & II ovarian cancer; 96 stages III & IV ovarian cancer, 79 benign ovarian tumors, 93 healthy | Chemiluminescence assay | Diagnosis | 98% | 76% | 31 |
| Squamous cell carcinoma antigen (SCC-Ag) | Cervical | 188 serum samples; 138 cervical cancer, 50 healthy | Chemiluminescence assay | Prognosis | 54.9% | 82.1% | 67 |
| Carcinoembryonic antigen (CEA) | Cervical | 139 blood samples; 7 cervical intraepithelial neoplasia, 80 squamous cell carcinoma, 16 adenocarcinoma, 36 healthy | ELISA | Prognosis | 98% | 33% | 57 |
| Serum fragments of cytokeratin (CYFRA) | Cervical | 188 serum samples; 138 cervical cancer, 50 healthy | ECLIA | Prognosis | 68.2% | 65.2% | 67 |
Abbreviations: ECLIA, electrochemiluminescence immunoassay analyzer; ELISA, enzyme-linked immunosorbent assay; SELDI-TOF-MS, surface-enhanced laser desorption/ionization.
Tumor biomarkers establishment requires an in-depth understanding of the cellular processes and molecular mechanisms initiating cancer, particularly focusing on how little changes in regulatory genes or proteins can interrupt a variety of cellular functions. The abundance of research conducted in gynecological cancer have revealed several diagnostic biomarkers that can potentially impact patient outcome. However, acceptance for routine use by regulatory bodies and clinical practice guidelines are lacking. Some of the crucial factors necessary for approval include specific analytical and clinical measurement criteria including cut-off value and rates of false-positive/negative of biomarkers. Besides, the biological justification for use of the biomarkers, including a complete understanding of the underlying pathway of the disease process, and how the biomarker is involved in the disease pathway are also important.
Despite the obstacles and limitations at present, the advancement in biomarker studies holds promise for the transition into clinical practice soon. For instance, the application of high-throughput proteomics technologies in large-scale experiments has overcome the difficulty of analysing low abundance cancer-derived proteins in body fluids. Furthermore, an acceleration in the identification of novel biomarkers is expected with advancements in artificial-intelligence-based bioinformatics tools. Although several validated single biomarkers lack an advantage over routine biomarkers such as B7-H4 biomarker for diagnosis of early OC when compared to CA125,30 current data recommends the incorporation of a multi-biomarker panel for superior sensitivity and specificity. It is predicted that more affordable mass spectrometry-based diagnostics will be employed in pathology laboratories, providing opportunities for cost-effective and robust proteomic profiling for better detection, prognosis, and management of cancer patients. Therefore, investment and funding of multicenter clinical trials to validate the efficacy of the most promising biomarkers is timely. We propose validation studies for multi-biomarker panels of ApoA1+CA125+transthyretin and VCAM-1+CA125+CEA+HE4 for early diagnosis of ovarian cancer. For enhanced prognostic value, we suggest combination panels of CA125+HE4 for endometrial cancer, and SCC-Ag+CEA for cervical cancer.
Future biomarkersIn recent times, there is exponential research exploring nucleic acids as novel serum markers, yielding higher specificity and sensitivity compared to protein biomarkers.59 MicroRNAs (miRNAs) are the most studied and play a crucial role in regulating the expression of their target mRNAs to assist tumor growth, invasion, angiogenesis, and immune evasion.60 High expression levels of several miRNAs such as miR-200a, miR-200b, miR-200c and miR-373 have been found to be associated with ovarian cancer progression.61,62 The long noncoding RNAs (lncRNAs) are also considered to play an important role in the occurrence and development of tumors. The interaction between microRNAs and lncRNAs has been of research interest lately. Jin et al., reported the lncRNA SNHG12 promoted progression in cervical cancer by sponging miR-125b.63 In addition to the above mentioned, circular RNAs (circRNAs) that can regulate gene expression and influence cellular activities such as cell proliferation, cycle progression, cell senescence, and apoptosis have shown promise in various types of cancer.64 It is noteworthy to mention that circRNAs have been identified as emerging prognostic biomarkers and as potential therapeutic tools to treat gynecological cancers.65
ConclusionAlthough many potential protein tumor biomarkers have been identified over the years, most have not translated into routine clinical practice, apart from CA125 and HE4 for ovarian cancer. The benefit of multi-biomarker panels, maximising affordable high-throughput proteomics technologies coupled with bioinformatics in larger study cohorts will be effective in validating previous reports, and to deliver more reliable and reproducible results.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Ethics approvalNot applicable.
Informed consentNot applicable.
FundingThe work was funded by the Ministry of Higher Education Malaysia for Fundamental Research Grant Scheme with project code: FRGS/1/2019/SKK13/USM/01/1.
Conflict of interestNone.