The ideal ratio between liver graft mass and recipient body weight for liver transplantation in small infants is unknown; however, if this ratio is over 4%, a condition called large-for-size may occur. Experimental models of large-for-size liver transplants have not been described in the literature. In addition, orthotopic liver transplantation is marked by high morbidity and mortality rates in animals due to the clamping of the venous splanchnic system. Therefore, the objective of this study was to create a porcine model of large-for-size liver transplantation with clamping of the supraceliac aorta during the anhepatic phase as an alternative to venovenous bypass.
METHODFourteen pigs underwent liver transplantation with whole-liver grafts without venovenous bypass and were divided into two experimental groups: the control group, in which the weights of the donors were similar to the weights of the recipients; and the large-for-size group, in which the weights of the donors were nearly 2 times the weights of the recipients. Hemodynamic data, the results of serum biochemical analyses and histological examination of the transplanted livers were collected.
RESULTSThe mortality rate in both groups was 16.5% (1/7). The animals in the large-for-size group had increased serum levels of potassium, sodium, aspartate aminotransferase and alanine aminotransferase after graft reperfusion. The histological analyses revealed that there were no significant differences between the groups.
CONCLUSIONThis transplant method is a feasible experimental model of large-for-size liver transplantation.
At present, liver transplantation is considered the gold standard treatment for terminal hepatic diseases in children (1). Advances in surgical techniques, intensive care and immunosuppressive therapy allow this complex procedure to be performed in younger and smaller infants.
The ideal ratio between the liver graft mass and recipient body weight (GBWR) is not known, but it is believed that the graft must weigh approximately 0.8 to 2.0% of the recipient's weight (2). However, in clinical practice, the left lobe or the left lateral segment of a graft from an adult donor transplanted into an infant weighing less than 10 kg may exceed this ratio.
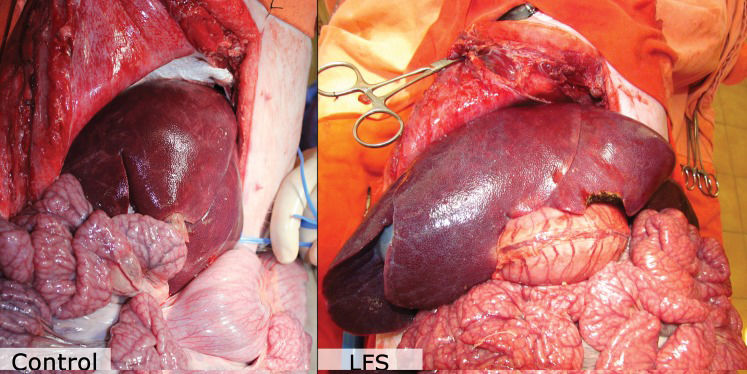
When this ratio is over 4%, a condition called large-for-size liver transplantation may occur. This condition is caused by the discrepancy between the small abdominal cavity and the large graft and is characterized by diminished blood supply to the liver graft with consequent hepatic dysfunction (3).
Some experimental models have been utilized to investigate the pathophysiological aspects of liver transplantation. Pigs are among the most frequently used animals for these models (4,5). However, in pigs, clamping of the venous splanchnic system during orthotopic liver transplantation (OLT) leads to high morbidity and mortality rates. Therefore, the use of venovenous bypass (VVB) is advocated, although this procedure can also cause complications (6,7,8). Another surgical maneuver that results in excellent hemodynamic stability is the clamping of the supraceliac portion of the aorta during the anhepatic phase. This maneuver is tolerated well in the pig liver transplant model (9).
Large-for-size experimental models have not yet been described in the literature. Therefore, the objective of this investigation was to create a porcine model of large-for-size liver transplantation with clamping of the supraceliac aorta during the anhepatic phase as an alternative to VVB.
MATERIALS AND METHODSThe experiments were performed in accordance with the guidelines of the National Institutes for Health for the care and use of laboratory animals. Fourteen Landrace-Large white pigs (weight, 17 to 20 kg) underwent OLT with whole-liver grafts and were divided randomly into two experimental groups according to the donor size:
- 1 -
Control group (n = 7); the weights of the donors were similar to the weights of the recipients (17 to 20 kg);
- 2 -
Large-for-size group (LFS- n = 7); the weights of the donors were nearly 2 times the weights of the recipients (40 to 50 kg)
After starvation for a period of 12 hours, the donors and the recipients were anesthetized with 5 mg/kg of propofol and maintained on a continuous infusion of intravenous fentanyl at a dose of 0.01 mg/kg/h and 1.5% inhalational isoflurane.
After an open cut-down of the right carotid sheath, a double lumen catheter was placed in the internal jugular vein for fluid administration. A single lumen catheter was inserted in the carotid artery for arterial pressure monitoring.
A total volume of 300 mL of blood was collected from the donors before liver perfusion; this blood was transfused into the recipient pig when necessary.
Donor procedureTo perform the donor operation, a long longitudinal midline thoracic and abdominal incision was performed for wide exposure of all of the organs. The portal vein, infrahepatic inferior vena cava (IHVC) and suprahepatic inferior vena cava (SHVC) were dissected. The hepatic artery was kept in continuity with the celiac trunk and abdominal aorta up to the iliac bifurcation. In situ cold liver perfusion (with Euro Collins® and Ringer's lactate solutions) was then performed through both the portal vein and the aorta. The harvested graft was weighed, immersed in a plastic bag filled with Euro Collins® perfusion solution at 4°C and placed in a basin. The back-table procedure was then performed.
Recipient procedureThe values for mean arterial pressure (MAP) and heart rate (HR) were registered at 4 standardized times as follows: at the beginning of the procedure (b), during aortic clamping (ao), 10 minutes after reperfusion (r) and immediately before sacrifice (s). Total hepatectomy was performed according to the classical description, and the supraceliac aorta was dissected and repaired near the diaphragmatic crura to allow for clamping during the anhepatic phase.
The graft was put into place, and the suprahepatic inferior vena cava, infrahepatic inferior vena cava and portal vein were then sutured in sequence. All anastomoses were performed using continuous sutures of 5-0 or 6-0 prolene. After completion of the venous anastomoses, the liver graft was reperfused. The recipient hepatic artery was anastomosed to the donor celiac trunk patch with continuous sutures of 8-0 prolene. The biliary reconstruction consisted of a choledocho-choledocho stented anastomosis (Figure 1.
Blood and serum analysisBlood was sampled in the recipient pigs at baseline and at 1 and 3 hours after portal reperfusion. In these samples, the serum levels of sodium, potassium, pH, bicarbonate, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined.
Tissue analysisThe hepatic tissue was sampled at baseline in the donors and at baseline, 1 hour and 3 hours after portal reperfusion in the recipients. The biopsy samples were preserved in a buffer solution with 10% formaldehyde for a period of 24 to 48 hours and were then embedded in paraffin. A semi-quantitative histological examination was performed in 4-µm-thick sections for all of the hematoxylin- and eosin-stained liver samples. Two independent investigators examined all of the tissue sections in a blinded fashion. The following data were analyzed: centrilobular necrosis, sinusoidal neutrophil infiltration, steatosis, apoptotic bodies and sinusoidal dilatation. For each of these parameters, the following scoring system was used:
0 - absence; 1 - mild; 2 - moderate, 3 - intense; 4 - very intense.
Statistical analysisThe mortality rates in the groups were expressed as percentages. The other results were expressed as the means±SD. For statistical purposes, the Kruskall-Wallis and Dunn tests were employed. A p-value <0.05 was considered to be significant.
RESULTSThe donor and recipient weights, graft-to-recipient body weight ratio and total and warm ischemia times are presented in Table 1. There were no differences between the control and LFS groups with regard to the recipient weight and the total and warm ischemia times. Donor weight and GBWR were significantly different between the groups (p<0.01).
Anthropometrical data of the animals and ischemia times during liver transplantation.
| Control | LFS | p-value | |
|---|---|---|---|
| Receptor (kg) | 20.66±1.38 | 20.20±1.92 | 0.67 |
| Donor (kg) | 19.66±2.13 | 44.88±6.21 | 0.01 |
| GBWR (%) | 3.06±0.42 | 6.30±0.74 | 0.01 |
| Total ischemia (min) | 138.80±17.77 | 147.00±8.26 | 0.37 |
| Warm ischemia (min) | 46.80±2.68 | 44.80±3.7 | 0.35 |
The mortality rate was 16.5% (1/7) in both groups. The death in the control group was related to bleeding from a laceration on the graft surface. In the LFS group, the only death was associated with hemodynamic instability immediately after aortic unclamping.
Hemodynamic dataData for MAP and HR at the standardized times are presented in Table 2. No differences in these parameters were observed between the groups.
Hemodynamic data during the experimental liver transplantation.
| Control | LFS | p-value | |
|---|---|---|---|
| MAP b (mmHg) | 86.50±3.10 | 82.00±11.78 | 0.9 |
| MAP ao (mmHg) | 109.50±4.20 | 109.80±4.20 | 0.9 |
| MAP r (mmHg) | 65.20±2.50 | 64.00±3.20 | 0.5 |
| MAP s (bts/min) | 66.20±2.90 | 67.50±2.00 | 0.6 |
| HR b (bts/min) | 83.20±6.90 | 86.00±3.60 | 0.48 |
| HR ao (bts/min) | 118.30±7.90 | 112.30±8.0 | 0.5 |
| HR r (bts/min) | 130.00±7.40 | 123.80±14.50 | 0.77 |
| HR s (bts/min) | 130.50±8.20 | 136.00±43.00 | 0.48 |
MAP: mean arterial pressure; HR: heart rate; b: beginning of the procedure; ao: during aortic clamping; r: 10 min after reperfusion; s: before sacrifice.
Table 3 shows the sodium, potassium, pH, bicarbonate, ALT and AST levels over the course of the experiment for both groups. The animals in the LFS group had increased serum levels of K and Na 1 hour after graft reperfusion compared to the control group. The Na levels were increased 3 hours after reperfusion. Finally, significant increases in ASL and ALT were observed in the LFS group when compared to the control group.
Results of serum analyses.
| Control | LFS | p-value | |
|---|---|---|---|
| pH (0) | 7.40±0.09 | 7.43±0.03 | 0.62 |
| pH (1) | 7.07±0.04 | 7.11±0.10 | 0.56 |
| pH (3) | 7.04±0.04 | 7.04±0.11 | 0.90 |
| K (0)Na (0) | 4.10±0.60138.00±1.80 | 4.30±0.30137.70±0.50 | 0.720.57 |
| K (1 h)Na (1 h) | 5.20±0.40141.20±2.50 | 6.60±0.80136.70±1.70 | 0.030.02 |
| K (3 h)Na (3 h) | 5.10±0.80142.20±2.30 | 6.20±0.60137.00±1.70 | 0.120.02 |
| AST (1 h) (IU/L) | 337.60±133.31 | 1001.40±485.73 | 0.02 |
| AST (3 h) (IU/L) | 392.80±128.90 | 1341.20±622.81 | 0.01 |
| ALT (1 h) (IU/L) | 39.00±5.70 | 62.60±16.50 | 0.01 |
| ALT (3 h) (IU/L) | 42.20±13.50 | 70.80±20.10 | 0.03 |
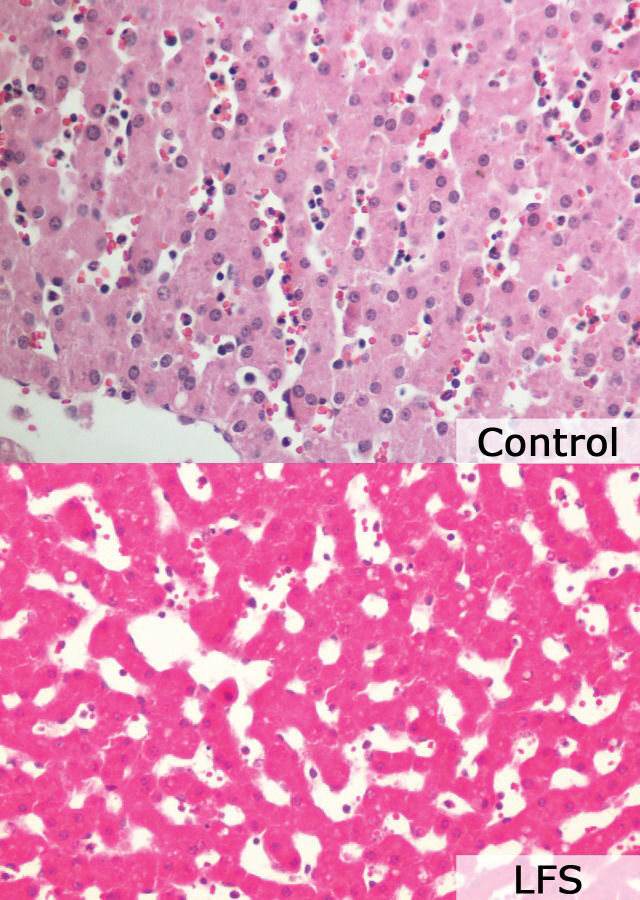
Table 4 presents the results of the histological analysis of the hepatic parenchyma performed using a semi-quantitative scoring system for histological features. There were no significant differences between the two groups (Figure 2.
Results of histological analysis. There were no differences between the LFS and control groups.
| Control | LFS | p-value | |
|---|---|---|---|
| Centrilobular necrosis | |||
| 1 h | 0 | 0.40±0.89 | 0.99 |
| 3 h | 0 | 0.40±0.89 | 0.99 |
| Sinusoidal neutrophils | |||
| 1 h | 2.00±1.00 | 1.80±0.84 | 0.97 |
| 3 h | 2.60±0.89 | 2.40±1.34 | 0.97 |
| Apoptotic bodies | |||
| 1 h | 0.20±0.45 | 0.40±0.89 | 0.99 |
| 3 h | 0.60±0.89 | 0.60±0.89 | 0.99 |
| Steatosis | |||
| 1 h | 1.20±1.30 | 0.20±0.45 | 0.28 |
| 3 h | 0.20±0.45 | 0 | 0.99 |
| Sinusoidal dilation | |||
| 1 h | 1.20±0.84 | 1.00±0.71 | 0.88 |
| 3 h | 1.20±1.10 | 1.40±0.89 | 0.58 |
The large animal model used in this study is straightforward, reproducible and clinically relevant. This model is the appropriate size with an anatomy similar to humans for the establishment and practice of new surgical techniques. Although small animal models (mice and rats, in particular) require a much smaller investment in material, personnel, space and time, they are not directly applicable to humans. Finally, the porcine animal model has the additional advantage of providing adequate conditions for the surgical training of young surgeons interested in liver transplantation.
Although there are many porcine models of size-matched and small-for-size liver transplantation (10,11,12), this is the first description of a large-for-size liver transplantation model. The exact early and late consequences of the relative hypoperfusion added to the ischemia-reperfusion lesion in liver allografts are not known.
Biliary atresia, the main indication for pediatric liver transplantation, usually leads to progressive hypoplasia of the portal vein and occasionally culminates in portal vein thrombosis (13,14). It is possible that this reduction in portal flow worsens the hepatocyte lesion in large-for-size transplants, which stresses the importance of creating a model to study the hepatic and hemodynamic repercussions of liver transplantation when the GBWR exceeds 4%.
One technical difficulty in porcine liver transplantation is the occasional lethal hemodynamic instability caused by simultaneous portal and caval clamping; to compensate for this, VVB is often used during the anhepatic phase. Pigs have a proportionally longer intestine and fewer spontaneous portosystemic communications than humans. Furthermore, they do not have an azygous vein, and the inferior vena cava enters the liver parenchyma in such a way that it precludes surgical procedures, such as the piggy-back implant. This explains why pigs do not tolerate simultaneous clamping of the liver pedicle and inferior vena cava (15) and why VVB is usually required during the anhepatic phase of OLT (16,17); however, VVB substantially increases the morbidity and complexity of the procedure.
Supraceliac aortic clamping during experimental OLT was first described in dogs by Isman H et al. in 1982 (18). In 1999, López-Santamaria et al. compared hemodynamic and biochemical variables in pigs subjected to liver transplantation with and without aortic clamping; none of the pigs in this study had VVB. They concluded that clamping of the supraceliac aorta during the anhepatic phase was tolerated well and resulted in excellent hemodynamic stability (9). In fact, in the present model, we confirmed the stability of hemodynamic data during transplantation (Table 2.
Aortic clamping considerably reduces operative time because VVB or construction of a mesocaval anastomosis is unnecessary. Aortic clamping also prevents splanchnic blood sequestration and the risk of reducing cardiac filling during clamping of the inferior vena cava.
There are potential harmful consequences of aortic clamping, which in clinical practice include pulmonary (19), renal (20), abdominal and spinal (paraplegia) complications (21). These complications are dependent on the level of aortic cross clamping (clamping of the thoracic aorta produces more complications than clamping of the infrarenal aorta), the duration and patient comorbidities (22). In our experiments using young, healthy pigs and an anhepatic phase lasting approximately 45 minutes, we believe that the complication rate should be low.
AST and ALT are commonly measured in the days following liver transplantation to evaluate hepatocellular injury. Although AST and ALT activity can be associated with injury of other organs (e.g., heart, brain and skeletal muscle for AST and heart and skeletal muscle for ALT), they are both considered to be good indicators of hepatocyte integrity (23). In fact, we observed that the AST and ALT levels were significantly higher in the LFS group compared to the control group at 1 and 3 hours after reperfusion. Considering that all of the other variables were similar in both groups (recipient weight and total and warm ischemia times), the likely cause for this result is the disproportion between blood flow and the hepatocyte mass. Indeed, the higher degree of hepatocyte destruction after LFS transplantation must be responsible for the significantly higher serum potassium levels in this group.
Kiuchi et al. described some anatomic, and even immunologic, disadvantages of LFS grafts (24), including a higher rate of vascular complications and more acute rejection episodes during the first month in LFS graft recipients. It is possible that the greater degree of hepatocyte ischemic destruction in LFS transplants causes a more intense exposure to allograft antigens and leads to the higher rate of acute rejection episodes.
Although the hemodynamic data were not altered by the size of the graft, the serum sodium levels were significantly lower in the LFS recipients. Because recipient resuscitation was achieved with Ringer's Lactate solution (sodium concentration, 130 mEq/L), it is possible that the fluid needed to maintain the same levels of arterial pressure is higher.
Histological analysis did not indicate any differences between the control and LFS groups, although we expected more intense ischemia-reperfusion injury in the LFS group. It is possible that this result occurred because of the short observation time in our study; if the biopsies were taken 24 and 48 hours after the procedure, these lesions may have been observed.
Finally, in contrast to the evidence in the literature and the conclusions reported herein regarding the large-for-size condition, Schulze et al. recently verified that the results of liver transplants in infants weighing ≤10 kg with GBWR of >4% were similar to infants with GBWR <4%. Moreover, based on their own results, the authors do not recommend the reduction of the left lateral segment transplantation for very small children (25).
In conclusion, in our original description of a pig experimental model of large-for-size liver transplantation, we demonstrated that supraceliac aortic clamping is a useful technical procedure that simplifies liver transplantation because it avoids the need to utilize VVB or a mesocaval shunt. Furthermore, this new model creates many research possibilities in this relatively new and unexplored area of pediatric liver transplantation.
AUTHOR CONTRIBUTIONSLeal AJ, Tannuri AC, Belon AR and Guimarães RR performed the experiments. Coelho MC, Gonçalves JO, Sokol SS and Melo ES performed the laboratory determinations. Tannuri U and Otoch JP performed the final revision of manuscript.
This project was funded by Fundação de Amparo a Pesquisa do Estado de São Paulo (project number 2011/12550-0).
No potential conflict of interest was reported.