The role of ovarian hormones and nitric oxide in learning and memory has been widely investigated.
OBJECTIVE:The present study was carried out to evaluate the effect of the nitric oxide synthase (NOS) inhibitor, N (G)-nitro-L-arginine methyl ester (L-NAME), on the ability of estradiol to improve learning in OVX rats using the Morris water maze.
METHODS:Forty rats were divided into five groups: (1) ovariectomized (OVX), (2) ovariectomized-estradiol (OVX-Est), (3) ovariectomized-L-NAME 10 (OVX-LN 10), (4) ovariectomized-L-NAME 50 (OVX-LN 50) and (5) ovariectomized-estradiol-L-NAME 50 (OVX-Est-LN 50). The animals in the OVX-Est group were treated with a weekly injection of estradiol valerate (2 mg/kg; i.m.). The OVX-LN 10 and OVX-LN 50 groups were treated with daily injections of 10 and 50 mg/kg L-NAME (i.p.), respectively. The animals in the OVX-Est-LN 50 group received a weekly injection of estradiol valerate and a daily injection of 50 mg/kg L-NAME. After 8 weeks, all animals were tested in the Morris water maze.
RESULTS:The animals in the OVX-Est group had a significantly lower latency in the maze than the OVX group (p<0.001). There was no significant difference in latency between the OVX-LN 10 and OVX-LN 50 groups in comparison with the OVX group. The latency in the OVX-Est-LN 50 group was significantly higher than that in the OVX-Est group (p<0.001).
CONCLUSION:These results show that L-NAME treatment attenuated estradiol-mediated enhancement of spatial learning and memory in OVX rats, but it had no significant effect in OVX rats without estrogen, suggesting an interaction of nitric oxide and estradiol in these specific brain functions.
Nitric oxide (NO) is a free radical gas that plays important physiological roles in biological systems.1 It acts as a diffusible intercellular signaling molecule in the central nervous system.2 NO is synthesized from l-arginine by NO synthase (NOS)3 and acts as a critical mediator in synaptic plasticity, long-term potentiation (LTP) and the consolidation of long-term memory.4-6 Several studies indicated that NOS inhibitors impaired memory consolidation7,8 and blocked LTP induction,9,10 while l-arginine, an eNOS (endothelial NOS) precursor, improved memory formation11 and reversed the effect of eNOS inhibitors.12
Previous studies have confirmed that ovarian steroid hormones affect learning and memory.13 Several experiments have shown that estradiol replacement after ovariectomy enhances memory in ovariectomized (OVX) rats. The enhancement might be due to the activation of cholinergic and aminergic systems; however, the exact mechanism is still unknown.14 It has been well documented that estrogen influences the NO system in both peripheral and nervous tissues.15-17 It has been shown that estrogen increases eNOS activity and expression18,19 as well as the production of nitric oxide in endothelial cells.20 There is evidence showing that estrogen affects nNOS (neuronal NOS) mRNA, the number of nNOS-expressing neurons and NO production in the brain regions such as hippocampus.21,22 Our previous study showed that the precursor of NO, l-arginine, affects the performance of OVX rats in the Morris water maze.23,24 Regarding the possible interaction between estradiol and NO systems,23,24 the aim of the present study was to elucidate the effects of N (G)-nitro-L-arginine methyl ester (L-NAME) (non-specific inhibitor of nitric oxide synthase) on learning and memory in OVX and estradiol-treated OVX rats using the Morris water maze test.
MATERIALS AND METHODSAnimals and drugsForty 8-week-old female Wistar rats (200±10 g) were obtained from the Razi Vaccine and Serum Research Institute of Mashhad. All rats were housed 4 per standard cage at room temperature (23±1°C) on a 12 h light/dark cycle with free access to water and food ad libitum. Rats were given one week to adapt to the new environment before any procedure was initiated. Animal handling and all related procedures were approved by the Mashhad Medical University Committee on Animal Research. L-NAME was purchased from Sigma Aldrich (St. Louis MO USA), and estradiol valerate was provided by Iran Hormone Pharm. (Tehran, Iran).
OvariectomyThe animals were ovariectomized under ketamine (150 mg/kg; i.p.) and xylazine (0.1 mg/kg; i.p.) 25,26 anesthesia. Anesthesia was confirmed by reduced respiratory rate and a lack of response to gentle pinching of the foot pad. A ventral incision was made through the flank skin of the rat, and the ovaries and ovarian fat were removed. Ovaries were isolated by ligation of the most proximal portion of the oviduct before removal.27
Groups and treatmentsAfter recovery, the animals were randomly divided into the following groups: (1) OVX, (2) ovariectomized-estradiol (OVX-Est), (3) ovariectomized-L-NAME 10 (OVX-LN 10), (4) ovariectomized-L-NAME 50 (OVX-LN 50) and (5) ovariectomized-estradiol-L-NAME 50 (OVX-Est-LN 50). The animals in the OVX-Est group were treated with weekly injections of estradiol valerate (2 mg/kg; i.m.).16,24 The OVX-LN 10 and OVX-LN 50 groups were treated with daily injections of 10 and 50 mg/kg L-NAME (i.p.), respectively. The animals in the OVX-Est-LN 50 group received weekly injections of estradiol valerate and daily injections of 50 mg/kg L-NAME. The animals in the OVX group received saline instead of L-NAME and estradiol valerate.16 All treatments were performed from the day after ovariectomy until the beginning of the behavioral study.
ApparatusTo assess visuospatial memory, rats were tested in the Morris water maze (MWM). The MWM is a black circular pool with a diameter of 136 cm and a height of 60 cm, filled with 20±1°C water to a depth of 30 cm. The maze was divided geographically into four equal quadrants, and release points (north, east, south and west) were designed at each quadrant. A hidden circular platform (10 cm in diameter) made of plexiglass was located in the center of the southeast quadrant and was submerged 1.5 cm beneath the surface of the water. Fixed, extra-maze visual cues were present at various locations around the maze (i.e., computer, MWM hardware and posters). An infrared camera was mounted above the center of the maze. An infrared LED was attached to each rat as a probe so that the animal's motion could be recorded and sent to the computer. A tracking system was used to measure the swimming speed, escape latency and travel path. 23,28
Behavioral assessmentAnimals received blocks of four trials in five daily sessions. During the 5-day period, the platform was situated in the center of the southeast quadrant and was submerged 1.5 cm below the surface of the water; therefore, it was invisible while testing spatial learning. The platform position was held constant during the 5-day period.
A trial was started by placing a rat into the pool facing the wall of the tank. Each of the four starting positions (north, east, south and west) was used once in a series of four trials, and the order of the starting position was randomized. Each trial was terminated as soon as the rat climbed onto the escape platform or when 60 s had elapsed. A rat was allowed to stay on the platform for 15 s. Then, it was taken from the platform, and the next trial was started after 20 s. Rats that did not find the platform within 60 s were put on the platform by the experimenter and were allowed to stay there for 15 s. After completion of the 4th trial, the animals were kept warm for an hour and were returned to their home cage.23,24,28 The latency and swimming speed to reach the platform were compared among the groups. All tests were conducted between 12:00 and 18:00.
Statistical analysisAll data were expressed as the means ± SEM. The data collected from different groups during the 5-day test period were compared using a repeated-measure ANOVA test with Tukey's post-test. Differences were considered statistically significant when p<0.05.
RESULTSThere was no significant difference between the latencies of the OVX-LN 10 and OVX-LN 50 groups in comparison with the OVX group; however, the effect of day was significant (Fig 1A)(treatment: DF = 2, F = 2.218, p = 0.1100; day: DF = 4, F = 26.04, P<0.0001) (interaction of treatment and days: DF = 8, F = 1.011, P = 0.4264). There was a significant difference when the latency was compared among the OVX, OVX-Est and OVX-Est-LN 50 groups. The effect of days was also significant (treatment: DF = 2, F = 15.83, P<0.001; day: DF = 4, F = 4.003, P<0.01; Fig 1A) (interaction of treatment and days: DF = 8, F = 0.75, P = 0.7493). Post hoc analysis showed that the animals of the OVX-Est group had a significantly lower latency to reach the hidden platform than the OVX group (P<0.01, Fig 1B); however, there was no significant difference between the OVX-Est-LN 50 group and the OVX group (Fig 1B). The latency of the first trial on day one was also comparable between the OVX and OVX-Est groups. The latency to reach the platform in the OVX group was 52.56± 3.91 sec, while in the OVX-Est group, it was 37.01±8.16 sec. There was no significant difference between the two groups when compared using an unpaired t-test (data not shown).
Comparison of the latency among the OVX, OVX-LN 10, OVX-LN 50, OVX-Est and OVX-Est-LN 50 groups. Data are presented as the mean ± SEM. (n = 8 in each group).The OVX-LN 10 and OVX-LN 50 groups were treated with daily injections of 10 and 50 mg/kg L-NAME, respectively, from the day after ovariectomy until the beginning of the behavioral study. The animals in the OVX-Est group were treated with weekly injections of estradiol valerate (2 mg/kg; i.m.). The animals in the OVX-Est-LN 50 group were treated with weekly injections of estradiol valerate (2 mg/kg; i.m.) and received daily injections of 50 mg/kg L-NAME. There were no significant differences in the latency between the OVX-LN 10 and OVX-LN 50 groups and the OVX group; however, the animals in the OVX-Est group had a significantly lower latency and time to reach the hidden platform than the OVX group (p<0.001). In the OVX-Est-LN 50 group, the latency was significantly higher than in the OVX-Est group (p<0.001). ***p<0.001 compared to OVX group; +++p<0.001 compared to OVX-Est group.
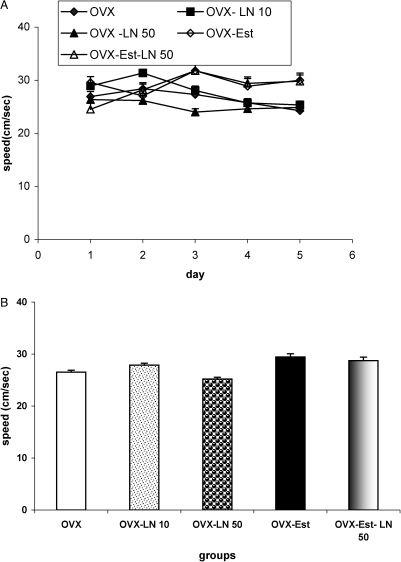
The swimming speed was also compared among the different groups, and the data are shown in Fig 2. There were no significant differences in swimming speed to reach the platform among the OVX, OVX-LN 10 and OVX-LN 50 groups; however, the effect of day was significant (Figs 2A and 2B). (treatment: DF = 2, F = 3.543, p = 0.06; day: DF = 4, F = 26.04, P = 0.0164) (interaction of treatment and days: DF = 8, F = 1.011, P = 2.155). There were also no significant differences in swimming speed among the OVX , OVX-Est and OVX-Est-LN 50 groups; however, the effect of day was significant (Fig 2A) (treatment: DF = 2, F = 3.218, p = 0.058; day: DF = 4, F = 26.04, P<0.001) (interaction of treatment and days: DF = 8, F = 1.011, P = 2.954).
Comparison of swimming speed among the OVX, OVX-LN 10, OVX-LN 50, OVX-Est and OVX-Est-LN 50 groups. Data are presented as the mean ± SEM. (n = 8 in each group). The OVX-LN 10 and OVX-LN 50 groups were treated with daily injections of 10 and 50 mg/kg L-NAME, respectively, from the day after ovariectomy until the beginning of the behavioral study. The animals in the OVX-Est group were treated with weekly injections of estradiol valerate (2 mg/kg; i.m.). The animals in the OVX-Est-LN 50 group were treated with weekly injections of estradiol valerate (2 mg/kg; i.m.) and received daily injections of 50 mg/kg L-NAME. There were no significant differences in swimming speed among the groups.
In the present study, the chronic effects of estradiol valerate, alone or in combination with L-NAME (the non-specific inhibitor of nitric oxide synthase), on learning and spatial memory of OVX female rats were investigated. OVX rats have been frequently used as a model of hormone deprivation to study post-menopausal changes in adult females.29,30 Therefore, we used this model to evaluate the effects of estradiol and L-NAME on the learning and memory of OVX rats. The results of the present study indicate that estrogen therapy in OVX rats improved spatial memory retention; the latency to find the hidden platform was significantly lower in the OVX-Est group than in the OVX group. This result was in agreement with that of Ping et al., who showed that treatment with estrogen significantly improved spatial learning performance in low-estrogen (OVX) rats.31 In a previous study performed in our lab, ovarian hormone depletion impaired spatial learning and memory,23,24 which was improved after treatment with estradiol.24 In contrast, Herlitz and co-workers showed that there were no considerable differences in cognitive performance between premenopausal and postmenopausal women.32 Other researchers also reported that estrogen had negative effects33,34 or no effects35,36 on learning and memory. It is unlikely that the estradiol result observed in this study was due to the effect of estrogen on motor capability because there was no significant difference among groups when swimming speed was compared. The discrepancies of the results regarding the role of estradiol on cognition may be due to the dose of estradiol, the route of administration, the duration of administration or the starting time of treatment.37-40 It has been shown that implantation but not oral delivery of 17 beta-estradiol has beneficial effects on spatial learning and dendritic spine densities in young ovariectomized rats.37 Animal and human studies have shown that there is a critical period after surgically induced menopause induced menopause during which estrogen is capable of increasing hippocampal function to a sufficient degree to enhance memory processing.41-44 After this period, estrogen replacement therapy is not successful in alleviating the hormone-related cognitive decline.45,46 It has been suggested that if treatment begins before the final menstrual period, hormone therapy has a beneficial effect, whereas initiation after the final menstrual period has a detrimental effect on cognitive performance.44 In the present study, treatment of the ovariectomized rats began immediately after removal of the ovaries. The results were consistent with those of Daniel and co-workers, who showed a beneficial effect of estradiol replacement on working memory in middle-aged rats when started immediately but not when started long after ovariectomy.47
Considering that the aging processes is an important effector in learning and memory,48,49,43 we used young female rats instead of middle-aged or aged animals, which have been frequently used by other researchers.50,51 The average lifetime of Wistar rats is about 30-31 months,52,53 and their puberty begins at 37-45 postnatal days.54-56 Therefore, the 8-week-old rats that were used in the present study were young animals. Learning and memory impairment due to ovariectomy and estradiol-mediated memory improvement in young animals has been frequently reported.57-59 The present study showed that removal of ovaries in young rats also impairs learning and memory and that this effect can be prevented by using estradiol.
The mechanism by which estrogen regulates spatial memory functions has been widely investigated. It has been reported that elevated levels of circulating estrogen in female rats result in increased spine and synaptic density and parallel increases in N-methyl-D-aspartate (NMDA) receptor binding in the CA1 area of the hippocampus.60,61 The increase in spine density is associated with increased sensitivity of CA1 pyramidal cells to NMDA receptor-mediated synaptic input,62 suggesting that the new spines and synapses induced by estrogen are enriched in NMDA receptors.63 There is also evidence showing that ovariectomy decreases the NMDA binding density in the hippocampal CA1 region and dentate gyrus, and that estradiol restores and increases NMDA binding density in the CA1 region.64 Estrogen also influences cholinergic neurochemistry in the basal forebrain and hippocampus, and it has been previously suggested that the ability of estrogen to alter NMDA receptor binding in CA1 is related to its ability to alter the cholinergic system.65 The NMDA receptor has been implicated in the induction of hippocampal long-term potentiation (LTP), and its highest density is in the hippocampus, which is associated with certain forms of learning.66
The relationship between NMDA receptors and the NO system in learning and memory has been widely investigated.2,67 It has been shown that the activation of NMDA receptors induces NO synthesis; consequently, NO plays a role in mechanisms of synaptic plasticity, including long-term potentiation (LTP) in the hippocampus.1 It has been widely reported that estrogen affects nNOS mRNA, the number of nNOS-expressing neurons and NO production in the brain regions such as hippocampus.21,22 Previous studies suggest that the function of estrogen in the central nervous system is related to increased nitric oxide production.15 Our previous studies showed that treatment of ovariectomized rats improves spatial learning and memory impairment caused by deletion of ovarian hormones.23 Later, we showed that nitric oxide probably contributes to the memory-improving effects of estradiol.24 The present study showed that chronic administration of L-NAME, in combination with estradiol, attenuated the estradiol-mediated improvement of learning in OVX rats; the latency of the animals treated with both estradiol and L-NAME in the MWM was significantly higher than that of the estradiol-treated group. Two doses of L-NAME, as administered previously by other researchers 68-70, were used; however, no effect was observed in the OVX rats. A dose of 50 mg/kg L-NAME was then used in combination with estradiol; L-NAME reversed the effect of estradiol. It seems that L-NAME has no effect on the learning procedure in ovariectomized rats when the NOS activity is low.71-75
This result confirms the contribution of estradiol and nitric oxide to spatial learning and memory.
Our results also indicate that the nitric oxide system might take part in memory formation in concert with some endocrine systems, including gonadal hormones, among which estrogen plays an important role.
The authors would like to thank the Vice Presidency of Research of Islamic Azad University, Khorasgan Branch of Isfahan, for financial assistance.