To elucidate independent risk factors for dysphagia after prolonged orotracheal intubation.
METHODS:The participants were 148 consecutive patients who underwent clinical bedside swallowing assessments from September 2009 to September 2011. All patients had received prolonged orotracheal intubations and were admitted to one of several intensive care units of a large Brazilian school hospital. The correlations between the conducted water swallow test results and dysphagia risk levels were analyzed for statistical significance.
RESULTS:Of the 148 patients included in the study, 91 were male and 57 were female (mean age, 53.64 years). The univariate analysis results indicated that specific variables, including extraoral loss, multiple swallows, cervical auscultation, vocal quality, cough, choking, and other signs, were possible significant high-risk indicators of dysphagia onset. The multivariate analysis results indicated that cervical auscultation and coughing were independent predictive variables for high dysphagia risk.
CONCLUSIONS:Patients displaying extraoral loss, multiple swallows, cervical auscultation, vocal quality, cough, choking and other signs should benefit from early swallowing evaluations. Additionally, early post-extubation dysfunction recognition is paramount in reducing the morbidity rate in this high-risk population.
Swallowing is a complex process that requires the precise timing and coordination of more than 25 muscles (1), including multiple oral-facial, pharyngeal, laryngeal, respiratory, and esophageal muscles (2), as well as 6 cranial nerves and frontal lobes (3). Alterations in this process, or dysphagia, can result in profound morbidity and can increase the probability of aspiration and delay proper oral nutrition administration (1). To prevent aspiration, a bolus of food or fluid reaching the posterior oral cavity stimulates neuroreceptors that trigger respiratory muscles to halt respiration, usually during exhalation (2-4).
It is no surprise that orotracheal tubes can disturb these intricately choreographed events and cause post-extubation dysphagia (2). Prolonged intubation, typically defined as an intubation lasting longer than 48 hours (3,5,6), is thought to contribute to swallowing dysfunction. The development of post-extubation swallowing dysfunction is well documented in the literature and occurs with a high prevalence, with 44 to 87% of these patients developing the condition (5,7). Factors that lead to post-extubation swallowing dysfunction are multifactorial and include oropharyngeal muscle inactivity, glottis injury, mucosal inflammation leading to the loss of tissue architecture, and vocal cord ulcerations. Additionally, the lingering effects of narcotics and anxiolytics can blunt protective airway reflexes (6,8). The clinical significance of post-extubation dysfunction is profound, as it can result in increased morbidity and mortality. Specific risk factors for these outcomes, however, have not been described for intensive care unit (ICU) patients who have received prolonged orotracheal intubation.
Various techniques have been developed to assess swallowing functions, including manometry, manofluorography, scintigraphy, electromyography, pH monitoring, and ultrasound analyses (5). Traditionally, videofluoroscopy has been considered the gold standard for swallowing evaluations (5,9,10). The clinical utility of this test is compromised, however, by the need to transport moderately ill patients to the radiology department, as well as the requirement of specialized equipment and personnel that are not readily available in many hospitals (11). Thus, screening protocols that are designed to identify patients at high risk for developing dysphagia are needed. These clinical screening procedures should be effective, based on the presence of specific symptoms, in determining which patients should undergo a more specific form of assessment.
Speech-language pathologists are trained to evaluate and treat oral motor function disorders objectively, manage facial and cervical muscle rehabilitation, and advise physicians regarding tube changes and the reintroduction of oral food intake (12,13). The aim of the participation of these professionals in multidisciplinary teams is to prevent and reduce complications resulting from oral motor function alterations (12,14,15), thereby reducing the length of hospital stay and readmission rates due to complications (16). Previous studies have already addressed the effectiveness of clinical swallowing assessment protocols (17). The clinical assessment sensitivity for predicting aspirations is still limited, however, because it remains difficult to detect all silent aspirations; therefore, speech pathologists must have reliable instruments when first evaluating post-orotracheal extubation patients (11).
The objective of this study was to elucidate the independent factors that predict dysphagia risk after prolonged orotracheal intubation (OTI) in ICU patients. Our hypothesis, based on the existing literature, was that clinical dysphagia predictors would include multiple swallows per bolus, limited laryngeal elevation during swallowing, and alterations in vocal quality after swallowing.
MATERIALS AND METHODSUsing the medical records from the Hospital das Clinicas da Faculdade de Medicina da Universidade de São Paulo, Brazil, we conducted a retrospective, observational cohort study on extubated ICU patients who had undergone a bedside swallow evaluation (BSE) by a speech pathologist. The study was approved by the Scientific and Ethics Committee of the Institution (CAPPEsq HCFMUSP 0224/10). Additionally, this study was approved as a retrospective document review; therefore, patient consent was not required.
Patient PopulationPatients were eligible if they met all of the following criteria: (a) the patient was admitted to an ICU (Instituto Central do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo) between September 2009 and September 2011; (b) the patient received prolonged intubation (>48 hours); (c) BSE was administered by a speech pathologist 24 to 48 hours following extubation; and (d) the patient was older than 18 years of age, (e) had clinical and respiratory stability, and (f) had a Glasgow Coma Scale score that was >14 points. The decision to consult a speech pathologist was left to the primary treating physician's discretion. Patients were excluded if they (a) were using a tracheostomy tube, (b) presented with neurological diseases, (c) presented with esophageal dysphagia, or (d) had undergone head or neck surgical procedures.
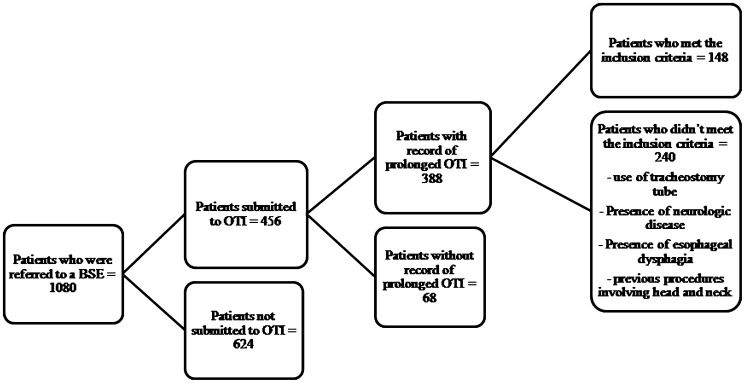
Of the 1,080 ICU patients who received a BSE, 456 had been subjected to an OTI; however, only 85% (388) had records of a prolonged OTI. Of the remaining patients, 148 met the inclusion criteria (Figure1).
In our hospital, the weaning and discontinuation of ventilatory support protocol are based on the American Association for Respiratory Care and the American College of Critical Care Medicine guidelines (18). These criteria are as follows: (a) evidence for some reversal of the underlying cause of respiratory failure; (b) adequate oxygenation (PaO2/Fio2 ratio >150 to 200, requiring positive end-expiratory pressure [PEEP] ≤5 to 8 cm H2O; Fio2 ≤0.4 to 0.5) and a pH ≥7.25; (c) hemodynamic stability, as defined by the absence of active myocardial ischemia and clinically significant hypotension (i.e., a condition requiring no vasopressor therapy or a low-dose vasopressor therapy, such as <5 µ/kg/min of dopamine or dobutamine); and (d) the capability to initiate an inspiratory effort.
Measurements: Clinical Swallowing AssessmentThe BSE included the application of the Dysphagia Risk Evaluation Protocol (DREP) (19), followed by the classification of the functional swallowing level according to the American Speech-Language-Hearing Association National Outcome Measurement System (ASHA NOMS) (20).
DREP (19) is a Brazilian bedside assessment protocol designed for early dysphagia risk detection. In our hospital, this is the standard protocol used to assess swallowing dysfunction in patients. DREP has already been validated (21) and includes items previously described as being effective in identifying high-risk dysphagia patients (13,22,23). It includes the controlled administration of water and puree/solid volumes. DREP determines whether a patient should receive larger volumes and different textures of food and liquids, as well as the amount of monitoring necessary for safe feeding. The protocol is divided into 2 sections, a water swallow test and a puree/solid swallow test, and the results are marked as either pass or fail for each of the observed items. As determined by the authors of the protocol, patient swallowing was assessed during the administration of 5 ml of water (via a syringe); 3, 5, and 10 ml of fruit puree (from a spoon); and half a piece of bread. The tests were repeated, if necessary, up to 3 times to confirm the results. The assessment procedures consisted of 11 items for the water swallow test and 12 items for the puree/solid swallow test. Patients were placed in the upright position so that their sitting position would not interfere with the research results (24). The assessed items and the criteria used to interpret the results are described below.
- A.
The Water Swallow Test (5 ml of water administered from a syringe)
- a.
Extraoral loss: Pass - Water does not escape from the patient's lips, and the patient manages the bolus adequately. Fail - The patient has difficulty managing the bolus, which causes drooling/spillage from the mouth.
- b.
Oral transit time: Pass - The patient swallows the bolus within 4 seconds. Fail - Patient takes longer than 4 seconds to swallow the bolus or does not swallow it.
- c.
Nasal reflux: Pass - Water does not escape from the patient's nasal cavities. Fail - Water comes out of the patient's nasal cavities.
- d.
Multiple swallows per bolus: Pass - The patient only needs 1 swallow per bolus. Fail - The patient needs more than 1 swallow per bolus, which causes drooling/spillage from the mouth. Additionally, the patient needs cues to complete the task.
- e.
Laryngeal elevation (monitored by positioning the index and middle fingers over the hyoid bone and the thyroid cartilage): Pass - The patient reaches an average elevation of 2 fingers. Fail - The patient does not present laryngeal elevation or presents an average elevation of less than 2 fingers.
- f.
Cervical auscultation (a stethoscope is placed at the lateral aspects above the cricoid cartilage and in front of the sternocleidomastoid muscle and large vessels): Pass - The patient presents the 3 characteristic sounds (two clicks followed by an expiratory sound), indicating that the bolus has gone through the pharynx. Fail - The patient does not present any sound or presents sounds other than those described above.
- g.
Oxygen saturation (baseline oxygen saturation is registered prior to the swallow test using a monitor or pulse oximetry): Pass - The patient does not present oxygen saturation changes of more than 4 units. Fail - The patient presents oxygen saturation changes of more than 4 units.
- h.
Voice quality: Pass - The patient does not present any alterations within the first minute after swallowing. Fail - The patient's voice becomes gurgly (“wet”) within the first minute after swallowing.
- i.
Cough: Pass - The patient does not cough within the first minute after swallowing. Fail – The patient coughs (voluntary or not) with or without throat clearing within the first minute after swallowing.
- j.
Choking: Pass - The patient does not choke after swallowing. Fail - The patient chokes during and/or after swallowing.
- k.
Other signs (cardiac and respiratory frequencies): Pass - The patient does not present significantly increased cardiac (60-100 beats per minute) and respiratory frequency (12-20 breaths per minute) changes. Fail - The patient presents signs of cyanosis, bronchospasm, and significant vital sign alterations.
- a.
The puree/solid swallow test (3, 5, and 10 ml of fruit puree offered from a spoon along with a half a piece of bread)
- a.
Extraoral loss: Pass - Water does not escape from the patient's lips, and the patient manages the bolus adequately. Fail - The patient has difficulty managing the bolus, which causes drooling/spillage from the mouth.
- b.
Oral transit time: Pass - The patient swallows the bolus within 20 seconds. Fail - Patient takes longer than 20 seconds to swallow bolus or does not swallow it.
- c.
Nasal reflux: The same as above.
- d.
Oral residue: Pass - An absence or up to 25% of the bolus residue is observed in the patient's oral cavity. Fail - More than 25% of the bolus residue is observed in the patient's oral cavity.
- e.
Multiple swallows per bolus: Pass - The patient requires 1-3 swallows per bolus. Fail - The patient requires more than 3 swallows per bolus, presents with drooling/spillage from the mouth, and needs cues to complete the task.
- f.
Laryngeal elevation: The same as above.
- g.
Cervical auscultation: The same as above.
- h.
Oxygen saturation: The same as above.
- i.
Voice quality: The same as above.
- j.
Cough: The same as above.
- k.
Choking: The same as above.
- l.
Other signs: The same as above.
- a.
The ASHA NOMS swallowing level scale is a multidimensional tool designed to measure both the supervision and diet levels required by assigning a single number between 1 and 7 (Table1). For this study, the patients' specific diets and supervision levels were used to calculate each patient's ASHA NOMS swallowing scale score. The speech-language pathologist was certified in assigning the ASHA NOMS swallowing levels. The ASHA NOMS level was determined based on the DREP results.
ASHA NOMS swallowing level scale.
| Level 1 | The individual is not able to swallow safely with their mouth. All nutrition and hydration is received through non-oral means (i.e., a nasogastric tube). |
| Level 2 | The individual is not able to swallow safely for nutritional and hydration purposes but may achieve some consistency with consistent maximal cues during therapy sessions only. An alternative feeding method is required. |
| Level 3 | An alternative feeding method is required, as the individual receives less than 50% of his/her nutrition and hydration by mouth, swallowing is safe with the consistent use of moderate cues to utilize compensatory strategies, and/or the patient requires maximum diet restriction. |
| Level 4 | Swallowing is safe but usually requires moderate cues to use compensatory strategies, the individual has moderate diet restrictions, and/or the patient still requires tube feeding and/or oral supplements. |
| Level 5 | Swallowing is safe with minimal diet restrictions and/or the patient occasionally requires minimal cues to use compensatory strategies. The patient may occasionally self-cue. All nutrition and hydration needs are met by mouth at mealtime. |
| Level 6 | Swallowing is safe, the individual eats and drinks independently, and the individual may rarely require minimal cueing. The individual usually self-cues when difficulty occurs and may need to avoid or requires additional time (due to dysphagia) to consume specific food items (e.g., popcorn and nuts). |
| Level 7 | The individual's ability to eat independently is not limited by altered swallowing functions. Swallowing is safe and efficient for all food consistencies. Compensatory strategies are effectively used when needed. |
Only the results obtained from the water swallow test were used for the analyses. The statistical analysis included assessment of the correlation between the water swallow test and the dysphagia risk level (i.e., ASHA NOMS). The purpose of this analysis was to identify which items (including extraoral loss, oral transit time, nasal reflux, multiple swallows per bolus, laryngeal elevation, cervical auscultation, oxygen saturation, coughing, choking, and other signs) were the most significant predictors of high dysphagia risk in the investigated population.
The variables were descriptively presented in contingency tables containing the absolute (n) and relative (%) frequencies. A logistic regression model was used to examine the relationships between the independent dysphagia risk variables. All variables were analyzed using a univariate model to determine statistical significance (p≤0.10). All significant variables and the interactions between them were used to obtain selections for a multivariate model (p≤0.05), according to the “enter” procedure. The variables that remained in the model were independent predictor variables.
RESULTSThe selected sample consisted of 91 males and 57 females, with a mean age of 53.64 years (range: 18-90 years). Patients presented an average of 1.08 OTIs (range: 1-2 OTIs) and an average of 182.4 hours of intubation (range: 48-720 hours). The average overall time required to perform the swallowing assessment following extubation was 36.0 hours (range: 24-48 hours).
Tables2) and 3) display the water swallow test results and the distribution of patients according to their ASHA NOMS levels, respectively. In the present study, the oxygen saturation and vital sign monitoring were recategorized as “other signs”. Additionally, for statistical purposes, the ASHA NOMS values were also recategorized, whereby L1 represented Levels 1 to 4 and L2 represented Levels 5 to 7.
The water swallow test results.
| Variables | Pass | Fail | ||
|---|---|---|---|---|
| n | % | n | % | |
| Extraoral loss | 130 | 88 | 18 | 12 |
| Oral transit time | 135 | 91 | 13 | 9 |
| Nasal reflux | 148 | 100 | 0 | 0 |
| Multiple swallows | 61 | 41 | 87 | 59 |
| Laryngeal elevation | 83 | 56 | 65 | 44 |
| Cervical auscultation | 99 | 67 | 49 | 33 |
| Voice quality | 127 | 86 | 21 | 14 |
| Cough | 84 | 57 | 64 | 43 |
| Choking | 128 | 87 | 20 | 13 |
| Other signs | 147 | 99 | 1 | 1 |
n – number of patients, % of patients.
Table4 presents the logistic regression model (univariate analysis) results, which were based on the independent dysphagia risk variables. For this analysis, ASHA NOMS Level 1 patients were considered a high dysphagia risk population; however, ASHA NOMS Level 2 patients were considered a low dysphagia risk population. The univariate analysis results indicated that the extraoral loss, multiple swallows, cervical auscultation, vocal quality, cough, and choking variables were possible significant high-risk indicators of dysphagia. Nasal reflux was not considered in this analysis because none of the patients failed the nasal reflux test. The “other signs” variable was also not considered because only 1 patient failed this item.
Logistic regression (univariate analysis) results based on independent dysphagia risk variables.
| Variables | Odds ratio | CI (95%) | p-value |
|---|---|---|---|
| Extraoral loss | 7.758 | 1.174–35.104 | 0.008* |
| Oral transit time | 11.143 | 1.409–88.106 | 0.022* |
| Nasal reflux | - | - | - |
| Multiple swallows | 2.164 | 1.111–4.218 | 0.023* |
| Laryngeal elevation | 1.750 | .902–3.394 | 0.098 |
| Cervical auscultation | 26.833 | 7.784–92.504 | <0.001* |
| Vocal quality | 20.968 | 2.731–160.978 | 0.003* |
| Cough | 42.273 | 13.752–129.939 | <0.001* |
| Choking | 19.603 | 2.548–150.839 | 0.004* |
| Other signs | - | - | - |
CI – confidence interval, *significant results.
Table5 presents the logistic regression model (multivariate analysis) results of the independent variables associated with dysphagia risk. According to these results, cervical auscultation and cough were independent predictive variables of high dysphagia risk.
A logistic regression (multivariate analysis) of the independent variables associated with dysphagia risk.
| Variables | Odds ratio | CI (95%) | p-value |
|---|---|---|---|
| Extra oral loss | 1.837 | 0.192–17.574 | 0.598 |
| Multiple swallows | 2.056 | 0.698–6.059 | 0.191 |
| Cervical auscultation | 12.709 | 2.940–54.931 | 0.001* |
| Vocal quality | 9.115 | 0.935–88.853 | 0.057 |
| Cough | 14.817 | 3.444–63.740 | <0.001* |
| Choking | 2.489 | 0.194–31.958 | 0.484 |
CI – confidence interval, *significant results.
This study represents the largest prolonged orotracheal intubation Brazilian patient group that has been screened for possible signs of dysphagia. To our knowledge, this is one of the few studies that have investigated possible dysphagia risk predictors based on clinical symptoms in ICU patients. Early post-extubation dysfunction recognition is paramount in reducing the rate of morbidity in this high-risk population.
Extended intubation durations have been correlated with dysphagia (5), and have also been reported to be independent predictors of dysphagia severity (1,28). Post-extubation, a higher dysphagia risk was reported in patients with Glasgow Coma Scale scores ≤14 (6) and in patients aged ≥55 years (1,6). In contrast, another study found that neither age nor intubation duration was correlated with increased swallowing dysfunction in post-orotracheal intubation patients (29). Prolonged intubation swallowing disorders extend the time before oral myofunctional/swallowing assessments can begin and the time to return to normal oral feeding while also delaying subsequent hospital discharges (27,28,30).
Screening procedures are generally designed to be quick (∼15 minutes), to be relatively non-invasive, and to provide little risk to the patient while identifying the dysphagia symptoms that may require an in-depth diagnostic assessment (23). In developing countries, the prolonged intensive medical and nursing care that is required by many patients places additional demands on already stretched healthcare budgets (31). Moreover, routine post-extubation swallowing studies can lead to additional, and possibly unnecessary, imaging, increasing healthcare resource use. Our results indicated that altered cervical auscultation and coughing during water swallow tests increased the likelihood of dysphagia in patients who underwent prolonged orotracheal intubation (11).
It is critical that health professionals distinguish between screenings and diagnoses. A screening does not define the nature of a patient's problem; it simply identifies the patient as being at risk for a significant problem/disorder (in this case, dysphagia) (23). The variables that were strong predictors of high dysphagia risk in our study are also considered to be variables associated with the possible presence of an aspiration (23,32,33). The reasons for aspirations have been discussed frequently in the literature, in which multiple causes have been postulated. It is known that alterations in the chemo- and/or mechanoreceptors (located in the pharyngeal and laryngeal mucosae) that are involved in the swallowing reflex are altered by the presence of an orotracheal tube (1). Inhibition of the sensory larynx abilities led to the absence of coughing or any other behavioral aspiration signs in patients following liquid bolus ingestion. Furthermore, this effect was observed immediately and 4 hours following extubation, and the detrimental effects were significantly reduced within 8 hours post-extubation (34). Additionally, most mucosal lesions caused by orotracheal tubes are healed 3 days following extubation (35).
Swallowing dysfunctions may persist, however, despite the removal of orotracheal tubes and the necessary spontaneous recovery periods. The swallowing dysfunction mechanisms following an extubation are thought to be a combination of muscle “freezing” (which may be attributable to the lack of swallowing while intubated) and the loss of proprioception (which may be attributable to mucosal lesions) (6).
Cervical auscultation is increasingly being used to supplement clinical swallowing assessments. The sounds associated with swallowing have been investigated using accelerometers and microphones to identify acoustic characteristics (36). Additionally, these sounds may also predict aspiration onset (37). The use of cervical auscultation varies with respect to its reported reliability (38) and validity compared with videofluoroscopic swallow studies (VFSS) (39,40). VFSS itself, however, has poor intra- and inter-rater reliability (41-43). According to Lazareck and Moussavi (37), swallowing sound assessments have great potential to reduce the need for VFSS and to assist in overall clinical swallowing assessments. It is clear from the literature that, despite the methods used to assess swallowing, providing the necessary training is indispensable.
A study by Bordon et al. (1) analyzed swallowing dysfunction risk factors after prolonged intubation in trauma patients. The authors also used a simple bedside speech pathology assessment (specifically, their swallowing failure indicators included coughing when drinking liquids and the presence of multiple swallows). Patients with and without post-extubation dysphagia were then compared by univariate analysis with respect to their age, the number of ventilator and ICU days, the presence or absence of pneumonia, and other variables. The authors suggested that older patients (above 55 years of age) with extended ICU stays and ventilator requirements may benefit from early swallowing evaluations. Similarly, Leder et al. (25) investigated aspiration incidence following extubation in critically ill trauma patients using a bedside transnasal fiberoptic endoscopic swallowing evaluation. The authors noted that aspiration was identified in 45% of the subjects, 44% of whom were silent aspirators. The authors argued that aspiration identification may reduce the likelihood of pulmonary complications following an extubation.
Although there are a few potential limitations of our study (e.g., it was a single-institution investigation and thus may only reflect local patient characteristics, and the study did not include any confirmatory fluoroscopic imaging to document silent or subclinical aspirations), our findings demonstrated that the overall swallowing deficit rates in patients subjected to prolonged orotracheal intubations (i.e., ASHA NOMS Level 1) were comparable to those that have been previously published. Studies performed with a direct laryngoscope indicated that approximately 56% of the observed critically ill patients displayed evidence of swallowing dysfunction (5,8). Similarly, studies performed with fiberoptic endoscopic evaluations (FEES) demonstrated that swallowing dysfunction occurred in approximately 52% of patients after prolonged intubation (29).
In the literature, there is no clear definition of which patients are at risk for dysphagia. The results of our study indicate that if patients who are subjected to prolonged orotracheal intubation present with altered cervical auscultation and coughing during a water swallow test, these patients should be given an early and more detailed swallowing assessment. Moreover, these assessments should possibly include imaging examinations before restarting oral nutrition. A similar study, performed with trauma patients subjected to mechanical ventilation (3), investigated whether a BSE could identify swallowing dysfunction in this patient group. One of the main findings of this study was that the patients who failed the BSE required longer mechanical ventilation than those who passed the BSE. Additionally, 78% of the patients intubated for more than 72 hours failed the BSE. All patients who passed the BSE, however, were discharged from the hospital without a clinical aspiration event. The authors also identified independent risk factors for BSE failures, which included tracheostomy, older age, prolonged mechanical ventilation, delirium tremens, traumatic brain injury, and spine fracture.
Finally, we would like to state that we did not study the long-term consequences of post-extubation dysphagia in our cohort because our end point was an evaluation performed within 48 hours of the observed extubations. Future studies at our institution will attempt to answer this and other questions.
AUTHOR CONTRIBUTIONSMedeiros GC was responsible for the data collection and analysis, interpretation of the results, and manuscript writing. Sassi FC organized and conducted the statistical analyses, interpreted the results, and wrote a major portion of the manuscript. Mangilli LD participated in the data collection and analyses and organized and conducted the statistical analyses. Zilberstein B was responsible for the medical criteria adopted in the experimental design and contributed to the data analysis and manuscript preparation. Andrade CR was responsible for the research and experimental design and contributed to the data analysis and manuscript preparation.
No potential conflict of interest was reported.