Chronic thromboembolic pulmonary hypertension is a disease affecting approximately 4,000 people per year in the United States. The incidence rate in Brazil, however, is unknown. The estimated survival for patients with chronic thromboembolic pulmonary hypertension without treatment is approximately three years. Pulmonary thromboendarterectomy for select patients is a potentially curative procedure when correctly applied. In Brazil, the clinical and hemodynamic profiles of chronic thromboembolic pulmonary hypertension patients have yet to be described.
OBJECTIVES:To evaluate the clinical and hemodynamic characteristics of chronic thromboembolic pulmonary hypertension patients scheduled for pulmonary thromboendarterectomy in a referral center for chronic thromboembolic pulmonary hypertension treatment in Brazil.
METHODS:From December 2006 to November 2009, patients were evaluated and scheduled for pulmonary thromboendarterectomy. The subjects were classified according to gender, age and functional class and were tested for thrombofilia and brain natriuretic peptide levels.
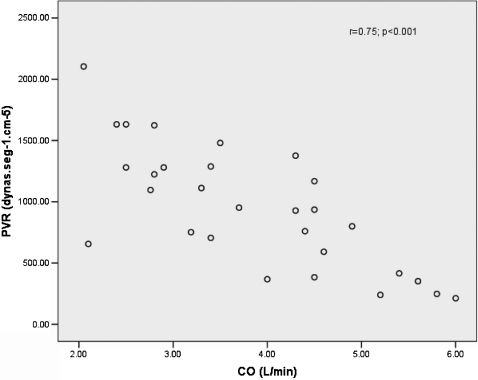
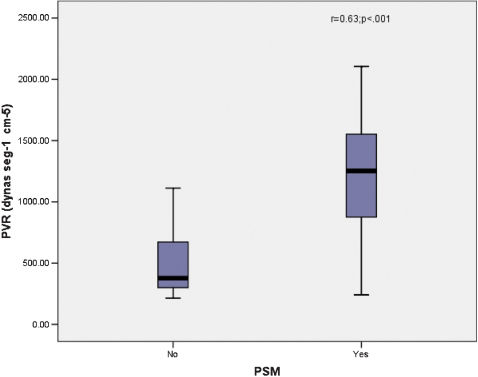
RESULTS:Thirty-five consecutive chronic thromboembolic pulmonary hypertension patients were evaluated. Two patients tested positive for schistosomiasis, and 31 were enrolled in the study (19 female, 12 male). The majority of patients were categorized in functional classes III and IV. Hemodynamic data showed a mean pulmonary vascular resistance (PVR) of 970.8 ± 494.36 dynas·s·cm-5 and a low cardiac output of 3.378 ± 1.13 L/min. Linear regression revealed a direct relation between cardiac output and pulmonary vascular resistance. Paradoxical septal movement was strongly correlated with pulmonary vascular resistance and cardiac output (p = 0.001). Brain natriuretic peptide serum levels were elevated in 19 of 27 patients.
CONCLUSIONS:In a referral center for pulmonary hypertension in Brazil, chronic thromboembolic pulmonary hypertension patients evaluated for pulmonary thromboendarterectomy had a hemodynamically severe status and had elevated brain natriuretic peptide serum levels. There was a predominance of females in our cohort, and the prevalence of hematological disorders and schistosomiasis was low (less than 10%).
Pulmonary arterial hypertension (PAH), defined as a mean pulmonary arterial pressure ≥ 25 mmHg at rest or > 30 mmHg during exercise,1 is a rare disease that leads to heart failure and death. There are many diseases that cause PAH, including idiopathic pulmonary arterial hypertension (IPAH). In addition, PAH is associated with anorexigen exposure, sleep apnea, collagenoses, chronic thromboembolic pulmonary hypertension (CTEPH) and schistosomiasis.1,2
Schistosomiasis is an important cause of PAH in the developing world.3,4 Unlike other countries, schistosomiasis-associated PAH (Sch-PAH) was found to be more prevalent than PAH associated with anorexigen exposure at a referral center for PAH in Sao Paulo.3,5
CTEPH is a disease that affects approximately 4,000 people per year in the United States, and its incidence rate is 1–5% within two years after the thromboembolic event.6–8 The incidence in Brazil is currently unknown; however, if the same numbers were used to forecast the incidence in Brazil, there would be around 1,525 new CTEPH cases each year in the country. CTEPH is caused by clot obstruction in pulmonary arteries, leading to an increased pulmonary vascular resistance (PVR) and pulmonary hypertension (PH).6,7 CTEPH is a severe disease characterized by persistent PAH after at least three months of an acute pulmonary thromboembolic episode.9 The average survival of patients with CTEPH without treatment is approximately three years. Pulmonary thromboendarterectomy (PTE) for select patients is a potentially curative procedure when correctly indicated,10 leading to a significant improvement in the quality of life.2 The success of PTE surgery is dependent on patient suitability. There is, however, no age restriction for this intervention.11,12
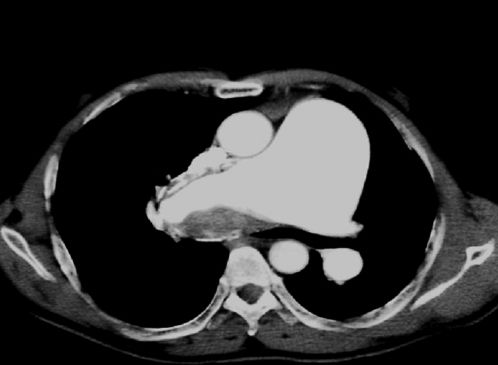
In Brazil, the clinical and hemodynamic profiles of CTEPH patients have yet to be described. PAH due to schistosomiasis is frequent and may be a confounding factor in the indication of PTE, given that PAH can sometimes be characterized by in situ thrombosis and the appearance of apparent clots in the pulmonary arteries on chest angiotomography (Figure 1). Important distal vessel remodeling, however, can be observed. Therefore, PTE indication may be incorrect for such patients because PAH will not decrease after surgical treatment, contrary to what is observed in the majority of subjects who undergo PTE. Because of these issues, it is important to establish a clinical and hemodynamic profile of CTEPH patients in Brazil who are scheduled for PTE.
The aim of this study was to evaluate the clinical and hemodynamic characteristics of patients with CTEPH who are scheduled for PTE at a referral center for treatment of this disease in Brazil.
METHODOLOGYThe present investigation was approved by the Research Ethics Committee of São Paulo Medical School, and informed written consent was obtained from all patients. This study was a transversal retrospective study with a prospective insertion.
From December 2006 to November 2009, patients with PAH were evaluated and scheduled for PTE. Thirty-five consecutive patients with CTEPH were eligible for PTE. Diagnosis of CTEPH was established by transthoracic echocardiography with Doppler, showing PH defined by a right ventricular systolic pressure (RVSP) > 35 mmHg, chest angiotomography, pulmonary angiography images compatible with thrombus and right-sided heart catheterization (RHC). Schistosomiasis was established by medical history, epidemiological characteristics and positive stool analysis for S. mansoni ova. Patients were classified according to gender, age and functional class.
Inclusion criteria consisted of operable CTEPH, characterized by central thrombotic lesions that are surgically accessible; a PVR > 300 dynas·s·cm-5 (or 3.75 Woods); a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg in the presence of a pulmonary capillary wedge pressure < 15 mmHg and a signed consent form.
Patients were excluded if they had a predominance of peripheral vascular obstruction on angiography; a creatinine clearance lower than 60 ml/min with a reduced or normal BNP level; or important comorbidities, including left heart failure, severe obstructive or restrictive pulmonary disease or neoplasia.
Clinical and functional evaluationA medical history was taken from each patient, followed by a physical examination. Demographic data, the presence of other diseases, the use of medications and the presence/absence of dyspnoea, hemoptysis, syncope, cough, thoracic pain, dizziness and palpitations were recorded. Patients were classified according to severity using the New York Heart Association functional classification (modified) (World Health Association), and a 6-minute walk test (6MWT) was performed based on the American Thoracic Society protocol.13
Hemodynamic measurements and acute vasodilator testingAfter clinical, functional and radiological evaluation, PAH patients were submitted to RHC according to a previously described protocol.14 Briefly, for RHC, a triple-lumen balloon-tipped catheter was inserted through the internal jugular vein (Swan-Ganz, model 131F7; Baxter, Edwards Critical Care Division, Irvine, CA). The pulmonary arterial pressure (PAP), pulmonary vascular resistance (PVR), pulmonary arterial occlusion pressure (PAOP) and cardiac output (CO) were measured. The indexed PVR (IPVR) and cardiac index (CI) were also calculated. Hemodynamic data were collected at baseline and after the administration of inhaled nitric oxide (NO) for 10 minutes. A complete hemodynamic status response was defined by a decrease in mPAP of at least 10 mmHg and an absolute decrease of mPAP under 40 mmHg in the presence of normal or increased CO during NO inhalation.
Laboratory testsLaboratory exams consisted of quantification of serum protein S and C, antithrombin III, factor V Leiden, anticardiolipin antibodies, lupus anticoagulant and antiphospholipid antibody and a determination of the presence of mutant prothrombin, homocystein and BNP serum. Creatinine clearance was also calculated. Stool sample analyses for S. mansoni ova were obtained.
Statistical analysisContinuous data are summarized as the mean and standard deviation (mean ± SD) or, in the case of a skewed distribution, as the median (25th percentile–75th percentile). Pearson correlation coefficients and regression analyses were calculated for the hemodynamic data. A p-value of < .05 was considered significant.
RESULTSPatientsThirty-five consecutive CTEPH patients were evaluated, and 31 were enrolled in the current study. Four patients were excluded, two who refused to be submitted to the RHC and two who tested positive for schistosomiasis. The characteristics of the patients enrolled are summarized in Table I, all submitted to clinical evaluation and haemodinamic studies, but the complete data were obtained in only 27 patients (technical limitations). Nineteen subjects were female, the majority of the patients were categorized in World Health functional classes III and IV, according to the diagnostic criteria defined by the World Health Organization.13,15 All patients had severe PH (Table I).
Baseline patient characteristics.
| Patients (n) | 31 |
| Gender (F: M) (n) | 19: 12 |
| Age, years∗ | 51.5 ± 13 |
| 6MWT, meters∗ | 346.9 ± 121.3 |
| Functional Class WHO (I–II): (III–IV) (n) | 10: 18 |
| Ecocardiogram findings | |
| RVSP, mmHg (n = 28)∗ | 83.6 ± 25.2 |
| EF, % (n = 30)∗# | 0.67 ± 0.06 |
| PSM (yes: no) (n = 27) | 18: 9 |
RVSP: right ventricle systolic pressure; EF: ejection fraction; PSM: paradoxical septal motion.
The hemodynamic data showed an increased PVR and low CO in the CTEPH patients (Table II). Twelve patients had a POAP higher than 14 mmHg. All patients had a negative acute reactivity test. There was a significant correlation between sPAP and RVSP (r = 0.44, p = 0.023).
Hemodynamic variables at baseline and after vasoreactivity testing.
| (n = 29) | Baseline | After NO inhalation |
|---|---|---|
| CO (L/min) | 3.378 ± 1.13 | 3.57 ± 0.87 |
| sPAP (mmHg) | 89.41 ± 26.32 | 83.36 ± 25.15 |
| dPAP (mmHg) | 32.89 ± 10.03 | 30.71 ± 12.5 |
| mPAP (mmHg) | 54.98 ± 15.33 | 50.14 ± 14.05 |
| PAOP (mmHg) | 15.86 ± 10.09 | 13.33 ± 4.84 |
| PVR (dynas·s·cm-5) | 970.8 ± 494,36 | 894,83 ± 439,04 |
CO: Cardiac output; sPAP: systolic pulmonary artery pressure; dPAP: diastolic pulmonary artery pressure; mPAP: mean pulmonary artery pressure; PAOP: pulmonary artery occlusion pressure; PVR: pulmonary vascular resistance. Data shown as the mean ± SD.
We found that 18 of 27 patients had paradoxical septum motion (PSM) as determined by echocardiogram. PVR had a strong correlation with PSM (r = 0.63; p = 0.001) (Fig. 3) and CO (r = -0.61; p = 0.001).
Laboratory findingsTwelve of 31 patients had hematological disorders, including the presence of factor V Leiden (n = 1), antithrombin III deficiency (n = 1), the presence of prothrombin mutation (n = 1), hyperhomocysteinemia (serum levels higher than 19 µmol/L) (n = 5), the presence of anticardiolipin and antiphospholipid antibodies (n = 3), the presence of the antinuclear factor (n = 3) and protein S deficiency (n = 1). None of the patients had protein C deficiency.
BNP serum levels were elevated in 19 of 27 patients [median: 138 (28–805) pg/mL], and BNP serum levels significantly correlated with the presence of PVR (r = 0.47, p = 0.018). BNP also had correlating trend with CO (r = -0.39, p = 0.053). No correlations were found between BNP and WHO functional class or 6MWT and RVSP.
DISCUSSIONIn the present study, we found a predominance of women with CTEPH, consistent with data described by Jamieson et al.16 Despite the fact that the hospital at which this study was carried out is a referral center for CTEPH, very few patients had Sch-PAH. One of the possible explanations for this finding is that this hospital is also a referral center for PAH associated with causes other than CTEPH, and only patients that have central clots in their pulmonary arteries are sent to our CTEPH service. The second reason is that two of the authors (MTF and FBJ) have given lectures at national meetings for over 10 years to draw attention to this little-known disease and to encourage other doctors to send their CTEPH patients to our specialized service.
The majority of patients were categorized in WHO functional classes III or IV, which is explained by the fact that patients with serious vascular diseases and signs of right heart failure are always recommended to referral centers.10 This often occurs too late because the disease is not well known, and the diagnosis is difficult. The presence of high RVSP and PSM in these patients confirms this finding. Patients with PAH have an increase in right ventricle (RV) pressure and prolonged isovolumetric contraction. As a consequence, the diastolic RV pressure becomes higher than in the left ventricle, leading the septum to move paradoxically. Mori et al.17 described PSM as a low cardiac index predictor. In this study, PSM was significantly correlated with CO and PVR, indicating that patients with PSM were hemodynamically more deteriorated.
In walking tests, it has been shown that 345 meters is the cut-off point to predict survival post-PTE.18 Our patients walked a mean distance of 347 meters. It is reasonable to believe that our patients will not have an excellent post-PTE prognosis, as along with their poor 6MWT scores, their mean PVR was greater than 970 dynas·s·cm-5. As indicated by Dartevelle et al., patients with a PVR lower than 900 dynas·s·cm-5 have a 4% mortality rate, whereas patients with a PVR between 900–1,200 dynas·s·cm-5 have a more than 10% mortality rate.19 In the present study, 33% of the patients had a PVR greater than 1,100 dynas·s·cm-5. This finding confirms that patients are typically referred in an advanced stage of disease, which may explain the high mortality rate found in this center (over 17%; data reflect patients not included in this research).
We also found that the patients had a hemodynamically severe status and had elevated PVR and low CO. This inverse relationship was significant and confirms previously published findings.20
Previous work by Skoro-sajer et al. showed that changes in mPAP during the NO vasoreactivity test are associated with better outcomes following surgery.21 In our study, however, none of the patients had a positive test. Similar to our results, Ulrich et al. found positive NO vasoreactivity tests in only 6% of CTEPH patients (1∶22 patients).22 It is possible that an increased number of patients could help explain the true significance of the NO vasoreactivity test in the prognosis for patients being submitted to PTE.
It was surprising to observe that the POAP was elevated in 12 patients, revealing the association of a postcapillary component. It has been hypothesized that CTEPH may be related to left ventricle diastolic dysfunction due to septum compression and hypertrophy caused by increased RV pressure or decreased LV filling.23,24
In addition, BNP or NT-proBNP serum levels have been used as markers of ventricular dysfunction in left heart disease and PAH.25–27 Nagaya et al.28 evaluated BNP serum levels in 34 CTEPH patients before and after PTA and found a significant correlation between BNP serum levels and PVR before and after surgery. Reesink et al.29 showed findings consistent with those of Nagaya et al., and the present study also showed that BNP is significantly correlated with PVR and has a trend to correlate with CO. These results suggest that BNP is a marker of severity in CTEPH, as observed in other studies.26–28
Hematological disorders are risk factors for CTEPH; however, the prevalence of these disorders is not high.30,31 Wolf et al. showed that the frequency of antiphospholipid antibodies in CTEPH was 20% in a population of 116 patients,32 similar to that found in our data.
The current study is not without limitations. For instance, our sample size was very small, part of our data was collected retrospectively, and our patients were not evaluated after PTE.
CONCLUSIONSThe CTEPH patients evaluated for PTE were predominantly female. The patients had a clinically and hemodynamically severe status and had elevated BNP serum levels. The prevalence of schistosomiasis and hematological disorders was low (less than 10%).
These findings lead us to deduce that patients arrive at our specialized center with a specific and advanced disease. We therefore emphasize the importance of an early and precise diagnosis to improve the survival of CTEPH patients by referring them before they reach an advanced stage.
CNPQ