The aim of the present study was to establish the frequency of metabolic disorders among patients with sudden deafness and to compare this frequency with data from population surveys.
INTRODUCTION:No consensus has been reached regarding the prevalence of metabolic disorders among sudden deafness patients or their influence as associated risk factors.
METHODS:This cross-sectional study enrolled all sudden deafness patients treated in the Otolaryngology Department of the University of São Paulo between January 1996 and December 2006. Patients were subjected to laboratory exams including glucose and cholesterol levels, low-density lipoprotein cholesterol fraction, triglycerides, free T4 and TSH.
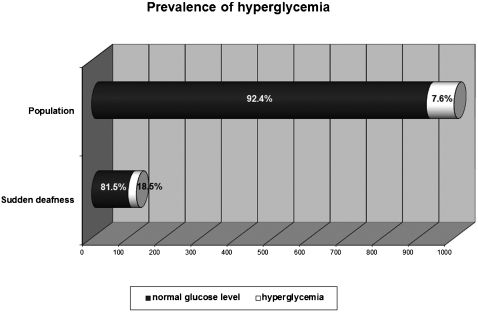
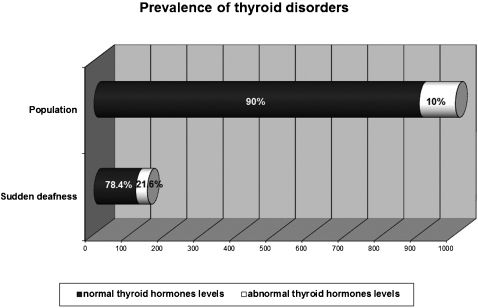
RESULTS:The sample comprised 166 patients. We observed normal glucose levels in 101 (81.5%) patients and hyperglycemia in 23 (18.5%) patients, which is significantly different (p < 0.0001) compared to the diabetes mellitus prevalence (7.6%) in the Brazilian population. Cholesterol levels were normal in 78 patients (49.7%) and abnormal in 79 (50.3%) patients, which is significantly different compared to the Brazilian population (p = 0.0093). However, no differences were observed in low-density lipoprotein cholesterol fraction (p = 0.1087) or triglyceride levels (p = 0.1474) between sudden hearing loss patients and the Brazilian population. Normal levels of thyroid hormones were observed in 116 patients (78.4%), and abnormal levels were observed in 32 (21.6%) patients. Compared with the prevalence of thyroid disorders in the general population (10%), statistical analysis revealed a significant difference (p = 0.0132) between these two groups.
DISCUSSION:Among sudden deafness patients, we observed frequencies of hyperglycemia and thyroid disorders that were more than twice those of the general population.
CONCLUSIONS:Hyperglycemia and thyroid disorders are much more frequent in patients with sudden deafness than in the general population and should be considered as important associated risk factors.
Sudden hearing loss (SHL) is defined as sensorineural hearing loss of greater than 30 dB over at least three contiguous pure-tone frequencies occurring within three days' period.1 Although many etiologies have been proposed for this disorder, the etiology can be identified in only 10 to 15% of cases.1 The inner ear can be directly affected by variations in serum insulin as well as by oscillations in plasma glucose levels.2 A health survey in the USA that employed a cross-sectional analysis of nationally representative data showed that hearing impairment was more prevalent among adults with diabetes than among those without.3 Previous studies have shown that auditory function can be affected by lipid metabolism, and serum cholesterol levels have been implicated as a significantly associated acquired risk factor in the SHL population.4-5 The effects of serum thyroid hormone levels have also been correlated with human auditory function; dynamic changes in hearing have been recorded during the development of hyper- and hypofunction of the thyroid gland.6 The incidence of deafness in cretinism is 35%, and hearing loss in hypothyroidism can be reversed with treatment of the disease.7
At our institution, among patients with cochlear-vestibular dysfunction and symptoms of dizziness and tinnitus, the prevalence of hyperglycemia ranges from 12 to 17%, that of hypercholesterolemia ranges from 46 to 57%, and that of abnormal thyroid hormone levels is around 15%. The prevalence of each of these disorders is significantly higher than that observed in the general population.8-10 No consensus has been reached regarding the prevalence of metabolic disorders such as diabetes mellitus, hypercholesterolemia, hypertriglyceridemia and thyroid dysfunction among SHL patients or their influence as associated risk factors. The aim of the present study was to determine the percentage of SHL patients who suffered from metabolic disorders at the time of hearing loss and to compare this frequency with that found in the general population as reflected by Brazilian population surveys.
MATERIALS AND METHODSThis cross-sectional study analyzed the records of patients treated in the Sudden Hearing Loss Section of the Otolaryngology Department of Clinic Hospital, Medical School of the University of São Paulo, between January 1996 and December 2006. The study protocol was reviewed and approved by the Research Ethics Committee (Research Protocol N0 1179/07, approved on April 7, 2008) and included all patients with SHL treated at the hospital. The inclusion criterion was sensorineural hearing loss of at least 30 dB in three or more consecutive frequencies that occurred with sudden onset over a 72-h period. All patients were subjected to a standard protocol that included collection of demographic data (sex and age), a test of hearing function, screening for the presence of vertigo and tinnitus, audiometric testing and imaging tests, including temporal bone magnetic resonance or computerized tomography. The laboratory exams included complete blood cell counts, glucose and cholesterol levels, LDL fraction, triglycerides, free T4 and TSH.
The following normal ranges were taken into account: fasting glucose 70 to 110 mg/dL, cholesterol up to 200 mg/dL, LDL up to 160 mg/dL, triglycerides up to 200 mg/dL, free T4 0.6 to 1.54 mg/dL, TSH 0.5 to 4.2 uU/mL. To assess the distributions of the studied parameters, we used the corresponding prevalences in the Brazilian population as reference data. Based on the 1988 National Census, the prevalence of diabetes mellitus is 7.6% among people aged 30 to 69 years.11 According to the Brazilian National Alert Campaign, the prevalence of hypercholesterolemia is 40% among adults older than 18 years (44.7 ± 15.7 years).12 Based on data from health care providers, the prevalence of dyslipidemia among adults aged ≥ 20 years is 30.4% for LDL levels greater than 160 mg/dL and 17.8% for triglyceride levels greater than 200 mg/dL.13 The rate of thyroid disorders is 10%.14
Statistical analysis included χ2 tests, independent samples t-tests for the differences between means by age group and determination of the confidence intervals (CI) for the differences between two proportions (e.g., frequency of metabolic disorders between SHL patients and the Brazilian population). A p value of less than 5% was considered significant.
RESULTSThe sample comprised 166 patients with SHL, 73 men (43.9%) and 93 (56.1%) women, mean age ± SD = 46.5 ± 16.2 years. The SHL was unilateral in 157 patients (94.6%) and bilateral in 9 (5.4%).
Testing of blood samples revealed normal glucose levels in 136 (82.4%) patients and hyperglycemia in 29 (17.6%) patients; one sample was lost. To homogenize our sample with the Brazilian survey,11 taking into account age data, we considered only SHL patients aged 30 to 69 years. Using these criteria, we observed 101 (81.5%) patients with normal glucose levels and 23 patients with hyperglycemia (18.5%) (Figure 1). Comparing this proportion with the historical reference value for diabetes mellitus prevalence among the Brazilian population, statistical analysis showed a significant difference in the frequency of hyperglycemia in patients with SHL (p < 0.0001, chi-square), with a difference between the two proportions of 0.1338 and a 95% CI of the difference between 0.0691 and 0.1984. Of the hyperglycemic patients, 14 were male and 9 were female, representing 11.2% and 7.2% of the SHL samples, respectively.
Total cholesterol levels were normal in 78 (49.7%) of the SHL patients, whereas hypercholesterolemia was observed in 79 (50.3%); nine samples were lost. Comparison between our sample and the patient sample in a Brazilian survey15 showed no significant difference in terms of mean age (p = 0.1579, unpaired t test) or standard deviation (p = 0.5565, unpaired t test). There was an increase on the prevalence of hypercholesterolemia in the SHL patient sample compared to the historical reference value for the Brazilian population. The statistical analysis showed a significant difference in the frequency of hypercholesterolemia in patients with SHL (p = 0.0093, chi-square).15 The difference between the two proportions was 0.0498, with a 95% CI of the difference between 0.0098 and 0.0899.
The LDL fraction was normal in 124 (79.5%) of the SHL patients, whereas hyperlipidemia was observed in 32 (20.5%); 10 samples were lost. To homogenize our sample with that used in the Brazilian survey13, taking into account age data, we considered only SHL patients aged more than 20 years. Within this group of patients, 118 (79.7%) had normal LDL levels and 30 (20.3%) exhibited LDL ≥ 160 mg/dL. Compared to the historical reference value for hypercholesterolemia prevalence among the Brazilian population,13 statistical analysis showed no difference in frequency between the two proportions (p = 0.1087, chi-square).
Normal levels of serum triglycerides were observed in 122 (79.2%) patients, and abnormal levels were observed in 32 (20.8%); 12 samples were lost. Again, to homogenize our sample with the Brazilian survey13, we considered only SHL patients aged more than 20 years; within this group, 115 (78.3%) patients had normal triglyceride levels, and 32 (21.7%) had triglyceride levels greater than 200 mg/dL. Compared to the historical reference value for hypertriglyceridemia prevalence among the Brazilian population,13 statistical analysis showed no difference in frequency (p = 0.1474, chi-square).
With respect to thyroid hormones, normal levels were observed in 116 (78.4%) patients, whereas 32 (21.6%) had abnormal levels; 18 samples were lost (Figure 2). Statistical analysis showed a significant difference in the frequency of thyroid disorders among patients with SHL and the historical reference value for thyroid disorder prevalence in the population (p = 0.0132), with a difference between the two proportions of 0.1988 and a 95% CI of the difference between 0.0359 and 0.3616. Of the patients exhibiting abnormal levels of thyroid hormones, 25 (78.1%) were female and 7 were male (21.9%). Subclinical hypothyroidism accounted for 17 cases (11.5% of the total SHL population), subclinical hyperthyroidism accounted for 13 cases (8.8%), and overt hypothyroidism accounted for 2 cases (1.3%).
DISCUSSIONOur study corroborates previous findings that among patients with SHL, mean age ranges from 43 to 53 years, gender distribution is even, and hearing loss is unilateral in over 95% of cases.16
According to data from the National Ministry of Health and a multicenter cross-sectional survey, the prevalence of diabetes mellitus and impaired glucose tolerance in the urban Brazilian population aged 30 to 69 years is 7.6% and 7.8%, respectively.11,17 The prevalence of diabetes mellitus is similar between females (7.6%) and males (7.5%) and increases with age, being 11.6% in the age group above 70 years of age and 0.1% in the age group below 30 years of age. Among SHL patients, we observed a significantly higher frequency of hyperglycemia, i.e., more than twice that expected for the Brazilian population. We also found that a greater proportion of males than females in the SHL population were affected by hyperglycemia, even within the patient age range used in the Brazilian survey.11 These data are in agreement with those of previous studies that show a higher frequency of glucose disorders in patients suffering from dizziness and/or tinnitus than in the general population.8-9 In a retrospective study of 148 idiopathic SHL patients, 16.2% of whom had type 2 diabetes mellitus, Fukui et al. found a similar frequency of hyperglycemia comparing to our own findings on the frequency within the SHL population.18
The mechanism by which hyperglycemia leads to sudden deafness is not well understood, but we can presume based on in vivo experimental studies that fluctuations in serum glucose lead to changes in serum osmolarity that interfere with outer hair cell motility and function, impairing cochlear microphonics.2,19 According to Lisowska et al., changes in cochlear micromechanics and in the retrocochlear auditory pathway are present as subclinical hearing impairment in diabetic patients.20 In a clinical trial using auditory brainstem responses (ABR), peripheral transmission time (wave I) and central transmission time (interpeak latencies I-V) were significantly delayed, and the mean amplitudes of the distortion product otoacoustic emissions (DPOAEs) were significantly reduced in diabetic patients presenting normal hearing compared with control subjects.20 We also know from in vitro studies that glucose utilization is high during cochlear development and that inner and outer hair cells are susceptible to oxygen and glucose deprivation.21-22 According to Amarjargal et al., loss of hair cells occurs during conditions of glucose deprivation, with inner hair cells being more vulnerable than outer hair cells.22 These data are also supported by epidemiological evidence from the American National Health Survey showing that hearing impairment is more prevalent among adults with diabetes (21.3%) compared to those without the disease (9.4%).3 A thirty-year national epidemiological survey that included four distinct samples analyzed the trends of sudden deafness in Japan.23 The authors observed that the number of SHL patients had increased more than eight-fold over the past 30 years (4,000 in 1972 versus 35,000 in 2001). The most significant finding of this study was that patients with sudden deafness exhibited diabetes mellitus more frequently in the most recent survey than in the first survey.23
In a Brazilian national survey of 81,262 individuals with a median age of 44.7 ± 15.7 years, a total cholesterol level greater than 200 mg/dL was observed in 40% of the population studied (38% of males and 42% of females); the frequency of elevated cholesterol was higher in older age groups.12 Among SHL patients, we observed a significantly higher frequency of hypercholesterolemia than that expected for the Brazilian population. However, for LDL fraction and triglyceride levels, the differences observed were not significant; thus, we do not consider these two prevalences to be different. Evidence from experimental studies demonstrates the influence of hyperlipidemia on hearing function.24-25 Experimental chronic hypercholesterolemia metabolically stresses inner ear tissue, inducing cochlear glycogen accumulation and edema in both the stria vascularis and outer hair cells.24 Ultrastructural morphological changes resulting from these conditions occur mainly in the basal cochlear turn and are associated with auditory dysfunction observed in ABR, in which reduced hearing sensitivity is observed in response to a cholesterol-supplemented diet.25
Vascular occlusion has also been suggested as a possible mechanism by which hyperlipidemia may contribute to an increased risk of SHL pathogenesis.5 In a clinical case-control study of 155 SHL patients and 155 controls, a multivariate analysis showed cholesterol levels to be an independent acquired risk factor 4.8 times more likely to be associated with the SHL population than with controls, indirectly supporting the vascular hypothesis.5 In a case-control study based on audiometric, ABR and transient-evoked otoacoustic emission (TEOAE) evaluations of patients suffering from hypercholesterolemia and hypertriglyceridemia, no significant differences were observed in mean auditory thresholds between the study and control groups.4 However, ABR evaluation showed prolonged latencies of the III and V waves, as well as prolonged I-III and III-V interpeak latencies, in the patients with hyperlipidemia in comparison with the control group.4 In another case-control study, the occurrence of hyperinsulinemia, diabetes mellitus, and hyperlipidemia was assessed in patients suffering from vertigo, tinnitus, or hearing loss of unknown origin; only hyperlipidemia seemed not to differ between patients and control subjects.26 With respect to hyperlipidemia, an opposite finding reported for the dizziness and tinnitus group implicates high levels of serum cholesterol in individuals with vestibular and cochlear complaints compared to the historical reference prevalence expected for the Brazilian population.8-9 In another clinical study, mean hearing levels in patients with chronic-phase SHL and hyperlipidemia, as diagnosed by serum cholesterol levels greater than or equal to 230 mg/dL, improved significantly after therapy with diet and the administration of antilipidemic agents.27 Although it is controversial in the literature, prevailing evidence seems to suggest that hyperlipidemia may have an influence on hearing function, although the data are not clear for SHL patients.
Subclinical hypothyroidism is defined as an elevated serum level of thyroid-stimulating hormone (TSH) and normal free thyroid hormone values. The prevalence of subclinical hypothyroidism in the American general population aged up to 12 years is 4 to 8%; the condition is significantly higher in females (5.8%) than in males (3.4%) and increases with age, especially beyond the age of 60.28 Subclinical hyperthyroidism is much less common than subclinical hypothyroidism; 3.2% of the population has TSH levels below 0.4 uU/L.29 In our sample, we observed a greater proportion of females than males affected by abnormal thyroid hormone levels. In addition, our sample as a whole exhibited a greater proportion of abnormal thyroid hormone levels than is reported for the general population. Taking into account that the prevalence of thyroid impairment in the general population is about 10%, we found a significantly higher frequency, more than twice as high as expected, in our sample.14 Similar results were obtained in a previous cross-sectional study of patients with dizziness.8 Taken together, these observations suggest that thyroid hormone levels may play a role in cochlear-vestibular function. In a multivariate linear regression study carried out using data collected over more than 20 years from 52 patients with SHL, Narozny et al. observed subclinical hyperthyroidism (low levels of TSH) in 15.4% of the cases, and this parameter was found to be a negative prognostic factor for hearing outcome.30 Although we found a somewhat lower frequency of subclinical hyperthyroidism in our sample, this difference may be due to the sample size. Our results agree with those of Narozny et al. in that the frequency of thyroid dysfunction may be two to four times higher among patients with SHL than that observed in the general population. In a case-control study with 109 patients, Nakashima et al. investigated risk factors for idiopathic SHL, and personal history of thyroid disease appeared to be significantly associated with SHL risk.31 Another multivariate linear regression study that analyzed two groups of SHL patients undergoing different treatments showed that normal thyroid function was a positive prognostic factor for hearing recovery.32
CONCLUSIONSHyperglycemia and thyroid disorders are much more frequent in patients with sudden deafness than in the general population and should be considered as important associated risk factors for sudden deafness.