Cardiovascular disease is one of the main causes of mortality and morbidity in diabetic patients. This study evaluated the effects of diabetes on myocardial capillary density and several serum angiogenic factors including nitric oxide, vascular endothelial growth factor, and soluble vascular endothelial growth factor receptors.
METHODS:Twelve male rats were divided into two groups: control and diabetic (n = 6 each). Diabetes was induced with a single dose of streptozotocin (50 mg/kg). After 21 days, capillary density in the myocardial tissue was evaluated using immunohistochemical staining and is reported as capillaries per mm2. Blood samples were collected before and after the induction of diabetes.
RESULTS:In the diabetic group, serum nitric oxide and soluble vascular endothelial growth factor receptor 2 concentrations were lower than the levels in the control group, while the level of soluble vascular endothelial growth factor receptor 1 was significantly higher. There was no significant change in the serum vascular endothelial growth factor concentration between the diabetic and control groups; however, the ratio of vascular endothelial growth factor to vascular endothelial growth factor receptor 1 was significantly lower in the diabetic animals. The myocardial capillary density was also lower in the diabetic group compared with the control group (1549±161 vs. 2156±202/mm2, respectively).
CONCLUSION:Reduced serum nitric oxide and vascular endothelial growth factor receptor 2 levels, increased serum vascular endothelial growth factor receptor 1 levels and a lower vascular endothelial growth factor to vascular endothelial growth factor receptor 1 ratio may be responsible for the decreased myocardial capillary density in diabetic rats.
Cardiovascular disease is one of the main causes of mortality and morbidity in diabetic subjects, and several long-term complications of diabetes involve impaired angiogenesis. Angiogenesis is the growth of new capillaries from pre-existing vessels1 and occurs during physiological conditions such as wound healing. However, abnormal angiogenesis may occur in some pathological conditions, including diabetic retinopathy.1
Nitric oxide (NO) and vascular endothelial growth factor (VEGF) are important factors affecting angiogenesis.2,3 NO has important roles in multiple physiological processes and pathological states,4,5 and VEGF and its receptors have essential roles in pathological and physiological angiogenesis.2,6 The two VEGF receptor subtypes that are primarily involved in angiogenesis are VEGF receptor type 1 (VEGFR-1) and VEGF receptor type 2 (VEGFR-2). Soluble VEGFR-1 (sVEGFR-1) binds with a high affinity to VEGF and reduces VEGF activity and angiogenesis,6–8 whereas VEGFR-2 has proangiogenic effect in the angiogenesis process.9 Increased VEGF-mediated angiogenesis in diabetic retinopathy and nephropathy and a decreased angiogenic response in wound healing and ulcers have been documented in diabetic subjects.10,11
This study aimed to evaluate the effect of diabetes on the serum concentrations of VEGF, NO and the soluble forms of VEGFR-1 and VEGFR-2 (sVEGFR-1 and sVEGFR-2) as well as myocardial capillary density in type I diabetic rats.
METHODSAnimalsMale Wistar rats (aged 10–12 weeks, weighing 230±30 g) were used in the present study. The animals were kept two per cage at room temperature (20–25°C) with a 12 h light/dark cycle and ad libitum access to food and water. The experimental protocols were approved by the ethics committee of Isfahan University of Medical Sciences.
Induction of DiabetesAfter one week of adaptation to the animal room, the rats were intraperitoneally injected with a single 50 mg/kg dose of streptozotocin (STZ, Sigma) to induce diabetes.12,13 STZ was dissolved in cold, normal saline and administered immediately. Two days after injection, the fasting blood glucose levels were determined. Animals with blood glucose greater than 300 mg/dl were considered diabetic.12,13 In the control group, normal saline was injected.
Experimental DesignThe rats were divided into normal and diabetic groups (n = 6 each). Blood samples were taken before and after the induction of diabetes. The samples were centrifuged, and the serum was kept at -70°C until the serum VEGF, NO, sVEGFR-1, and sVEGFR-2 concentrations were measured. After 21 days, the animals were euthanized. The apex of the heart was removed, washed with normal saline and fixed in formalin solution for immunohistochemical evaluation. Body weight was measured before induction of diabetes and 21 days after the experiment.
Serum VEGF, sVEGFR-1, sVEGFR-2, and NO MeasurementsSerum VEGF, sVEGFR-1, and sVEGFR-2 concentrations were measured using the quantitative sandwich enzyme immunoassay technique with the appropriate kits (R&D Systems, Minneapolis, USA).14,15 The serum nitrite concentration, one of the main metabolites of NO, was determined using the Griess reagent method (Promega, Madison, USA).16
ImmunohistochemistryCapillary density was measured using immunohistochemistry with a rat monoclonal antibody directed against mouse CD31 (Abcam, Cambridge UK).17 Fifteen fields of three different sections from each cardiac tissue sample were randomly examined using a light microscope (400×) by two blinded observers. The number of capillaries in each tissue sample is reported as the number of capillaries per mm2.
Statistical AnalysisAll values are reported as the mean ± SE. Student's t-test was used to evaluate differences between the two groups. Paired data were used for the data analysis before and after the induction of diabetes. Bivariate correlations were calculated using Pearson's correlation coefficient. A p-value less than 0.05 was considered statistically significant.
RESULTSBlood glucose and body weightTable 1 shows the fasting blood glucose levels and body weight in the two groups. Body weight significantly increased over the course of the experiment in the control group, whereas the body weight of diabetic rats decreased over time (p<0.05). The fasting blood glucose level in the diabetic animals was higher than the level in the controls and remained elevated throughout the experiment.
Effects of diabetes on serum angiogenic factorsFigure 1 illustrates the serum NO concentration in the two groups before and after the induction of diabetes. In the diabetic group, the serum NO level was significantly decreased at the end of experiment when compared with the level prior to the induction of diabetes (p<0.05; Figure 1). While there was no significant difference in the serum VEGF concentration between the groups (Figure 2A), the serum sVEGFR-1 concentration was increased and the sVEGFR-2 level was decreased in the diabetic group compared with the control group (p<0.05; Figure 2B and C). In addition, the VEGF:sVEGFR-1 ratio in diabetic animals was lower than the ratio in the controls (p<0.05; Figure 2D).
The capillary density in the myocardial tissue (expressed as the number of capillaries per mm2) was significantly reduced in the diabetic group compared with the control group (p<0.05) (Figure 3B). Representative images of the immunohistochemical staining are presented in figure 3A.
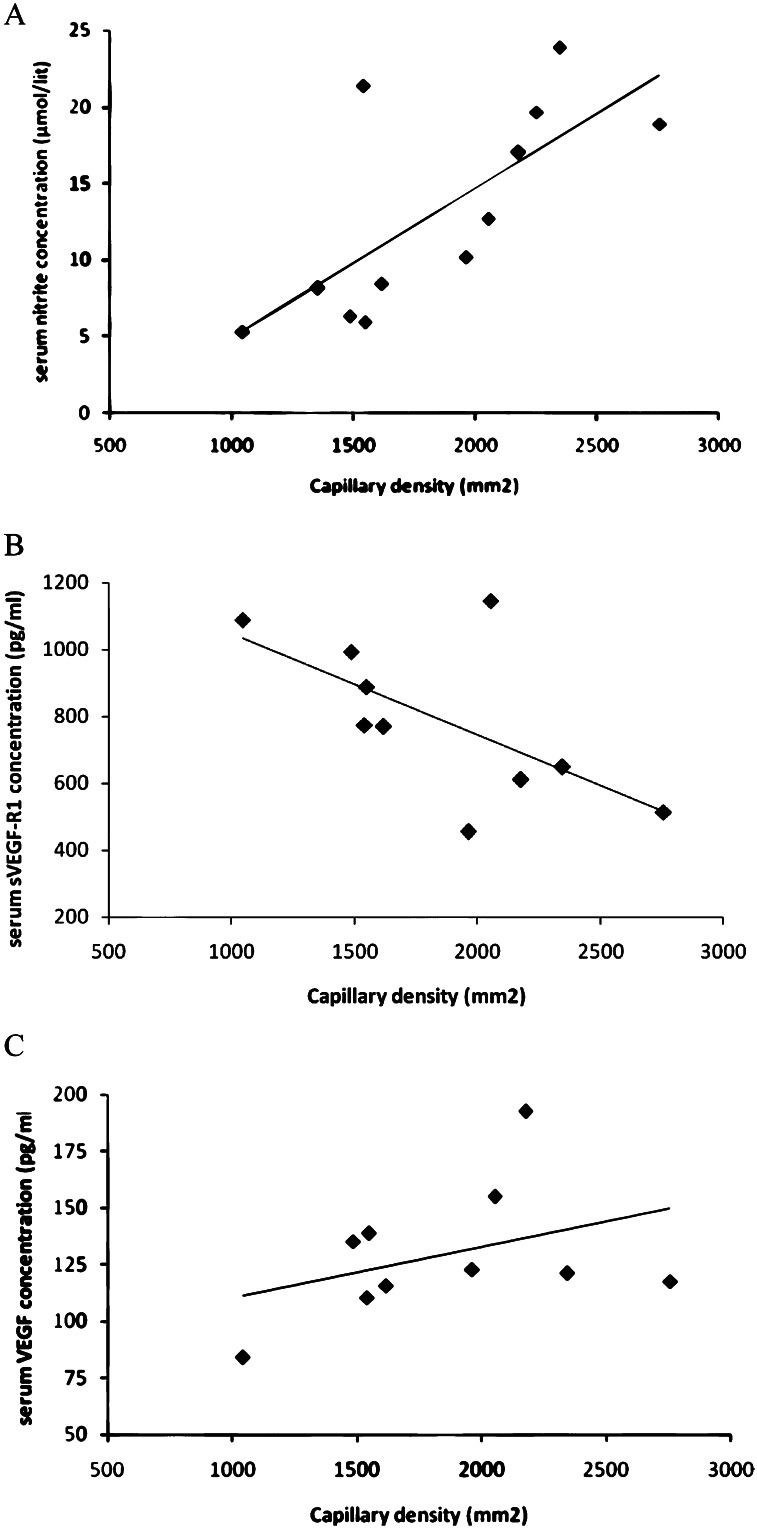
Correlation analysisIn the correlation analysis, we found that capillary density in myocardial tissue was positively correlated with the serum nitrite level (r = 0.70), the VEGF level (r = 0.40), the sVEGFR-1 level (r = -0.69) and the VEGF:sVEGFR-1 ratio (r = 0.71) (Figure 4).
DISCUSSIONOur results show that the capillary density in myocardial tissue, the serum NO level, and the sVEGFR-2 level in diabetic rats were lower than the levels in the controls, while the sVEGFR-1 level was higher in the diabetic animals. Moreover, the VEGF:sVEGFR-1 ratio was significantly decreased in diabetic animals. The correlation analysis demonstrated that capillary density in myocardial tissue was correlated with the above serum angiogenic markers. Angiogenesis is controlled by a number of proangiogenic and antiangiogenic factors that are released in tissues. NO and VEGF have crucial roles during angiogesis.6,18 NO not only has a direct effect on angiogenesis, but other angiogenic growth factors affect angiogenesis as well by increasing NO production.19 A decrease in NO production in endothelial NO synthase–deficient mice disrupts the development of the coronary vessels and angiogenesis.20 In the present study, the serum NO concentration in diabetic rats was lower than the concentration in the control group, which supports the results of previous studies.21–24 Some possible mechanisms responsible for decreased NO production under high glucose conditions include the suppression of endothelial NO synthase expression and activity,25 overproduction of superoxides,26 and activation of protein kinase C.23
VEGF has been shown to be an important inducer of angiogenesis in a variety of in vivo models.6 Several experimental and clinical reports examining the effect of diabetes on the serum or plasma VEGF level have been published, and the results are contradictory: an increase,27–29 decrease,30,31 and no change32,33 in the VEGF levels in type I and II diabetes have been reported. In the present study, although the capillary density was lower in diabetic animals, the serum VEGF concentration was similar to that in control animals. It has been suggested that even with an increase or no change in the VEGF level, the VEGF signaling pathway is defective during diabetes, and diabetes is considered VEGF resistant.29,34 In a recent study, Hazarika et al. showed that in the absence of ischemia, VEGF signaling in diabetic mice was lower than that in control mice.35 They also showed that in the absence of ischemia, diabetic mice had increased VEGF and sVEGFR-1 levels and decreased phospho-AKT/AKT and phospho-endothelial NO synthase/endothelial NO synthase levels. Lu et al. also found that the post-ischemic capillary density and revascularization in the limbs of diabetic rats was decreased, with reduced mRNA and protein expression of eNOS, VEGF, and bFGF.31 However, despite the similarity in serum VEGF levels between the groups, we found that diabetic animals had higher sVEGFR-1 and lower sVEGFR-2 levels compared with the controls. sVEGFR-1 binds with a high affinity to VEGF9 and reduces VEGF activity, endothelial cell proliferation, angiogenesis and tumor growth.6–8 Thus, sVEGFR-1 acts as an antagonist of VEGF.36 In contrast, VEGF-R2 is an effector of proangiogenic signaling in the angiogenesis process.9 Therefore, reduced sVEGFR-2 levels and increased sVEGFR-1 levels in the serum may be responsible for the lower capillary density in the myocardial tissue of diabetic rats. We also investigated the VEGF to sVEGFR-1 ratio, as some studies have used the VEGF:sVEGFR-1 ratio as an indicator of angiogenesis status.37,38 In the present study, we found that the VEGF:sVEGFR-1 ratio was reduced in diabetic rats, which suggests decreased angiogenesis in the diabetic group.
In conclusion, myocardial capillary density was lower in the diabetic group compared with the control group. Furthermore, the lower serum levels of the proangiogenic factors NO and sVEGFR-2, the higher level of the antiangiogenic factor sVEGFR-1 and the reduced VEGF:sVEGFR-1 ratio may explain the reduced coronary angiogenesis observed in diabetic animals.
This study was supported by a grant from Isfahan University of Medical Sciences (grant number: 188103).