To evaluate the expression of the cell adhesion molecules E-cadherin and N-cadherin and the transcription factor Snail in invasive ductal breast carcinomas and to determine their relationships with clinicopathological features.
METHODS:Immunohistochemistry was used to examine E-cadherin, N-cadherin, and Snail protein expression in 132 invasive breast carcinomas.
RESULTS:The expression of E-cadherin was decreased (negative or weak) in 37.1% of invasive carcinomas, while N-cadherin and Snail overexpression were detected in 51.9% and 40.9% of carcinomas, respectively. Low E-cadherin expression was significantly correlated with poorly differentiated carcinoma (53.1%), positive node status (80.9%), poor Nottingham Prognostic Index (64.7%), and the presence of estrogen and progesterone receptors. Overexpression of N-cadherin and Snail were also significantly correlated with poorly differentiated carcinoma, positive node status, and poor Nottingham Prognostic Index but were correlated with the absence of hormone receptors. Loss of E-cadherin immunoexpression was strongly associated with the presence of membranous N-cadherin (87.8%) and nuclear Snail (69.4%).
CONCLUSION:Loss of E-cadherin and overexpression of N-cadherin and Snail in breast carcinomas may play a central role in the development of invasive ductal breast carcinoma. These biomarkers may provide a valuable reference for the study of invasive ductal carcinoma progression and to characterize the biological behavior of the tumor. In the future, increased N-cadherin and decreased E-cadherin expression may be used as indicators of the progression and prognosis of invasive ductal carcinoma.
Breast cancer is the most common cancer among Egyptian women. According to the most recent (2007) registry of the Egyptian National Cancer Institute, breast cancer represents 34.26% of female cancers.1
Embryonic development, tissue remodeling, restitution and wound repair are key biological processes that require epithelial cells to escape from the rigid structural constraints of their tissue architecture and achieve cell migration and movement. The epithelial–mesenchymal transition (EMT) is a fundamental and highly conserved process that achieves this morphogenetic transformation.2,3 The progression of breast cancer is often accompanied by changes in the pattern of gene expression of neoplastic cells, resulting in a highly tumorigenic and invasive cell phenotype. Some of these changes are reminiscent of EMT, as they result in a loss of epithelial features and a gain of mesenchymal properties.4 In epithelial tumors, changes in the profile and activity of adhesion molecules accompany malignant progression.5 E-cadherin, the prototypical adhesion molecule expressed in epithelial cells, is frequently lost in epithelial malignancies, whereas N-cadherin, which is absent in normal epithelia, is up-regulated in many invasive tumors.5–8 In aggressive tumors, EMT is characterized by reduced E-cadherin and increased N-cadherin expression, contributing to a stroma-oriented cellular adhesion profile, increased tumor cell motility and other invasive properties.9 Thus, the localized occurrence of EMT allows the tumor to progress to invasive cancer and metastatic disease.10,11 In human breast cancer, N-cadherin is up-regulated in invasive ductal carcinomas and is further increased in tumors with metastatic potential.12
Snail belongs to the conserved Snail superfamily of transcription factors, which are expressed at different stages of development in different tissues.13 Recently, Snail has been implicated in the progression of human tumors via its regulation of E-cadherin.14 Specifically, Snail interacts with proximal E-boxes of the E-cadherin promoter to suppress its expression.15 Such transcriptional repression mechanisms have emerged as one of the crucial processes underlying the down-regulation of E-cadherin expression during development and tumor progression.
The main aim of this study was to evaluate whether E-cadherin, N-cadherin, and Snail expression could be used as markers of the progression of invasive ductal breast carcinoma. To this end, we investigated the correlations between the expression of these proteins and different clinicopathological variables in 132 cases of primary invasive ductal breast carcinoma.
MATERIAL AND METHODSFormalin-fixed and paraffin-embedded tissue blocks of tumor samples from 132 patients diagnosed with invasive ductal breast carcinoma (IDC) at the Pathology Department of Minia University Hospital between 2007 and 2009 were included in this study. All carcinomas were ductal not otherwise specified, and 54 were characterized as ductal carcinoma in situ (DCIS) associated with invasive carcinoma. All cases were reviewed by H. M. Abd Elmoneim to confirm the diagnosis. The characteristics of these retrospective cohorts are detailed in Table 1. Patients were only enrolled in the study if paraffin blocks suitable for immunohistochemistry were available and adequate clinical parameters had been collected. Histologic type and grade for invasive cases were defined according to the WHO and modified Scarf-Bloom-Richardson classifications, respectively.16 Tumor staging was performed according to the Tumor, Node, Metastasis (TNM) system of the International Union Against Cancer.17
Clinicopathological data of 132 invasive ductal breast carcinoma patients.
| Clinicopathologic characteristics | No. (%) n = 132 |
|---|---|
| Age (y)<50 years≥50 years | 52 (39.4%)80 (60.6%) |
| HistologyIDCIDC + DCIS | 78 (59.1%)54 (40.9%) |
| Tumor size≤2 cm2-5 cm>5 cm | 15 (11.4%)82 (62.1%)35 (26.5%) |
| GradingIIIIII | 10 (7.6%)58 (43.9%)64 (48.5%) |
| Lymph node statusNegative1-3 positive>3 positive | 51 (38.6%)31 (25.8%)47 (35.6%) |
| StagingIIIIII | 13 (9.8%)70 (53%)49 (37.2%) |
| NPIFavorableModeratePoor | 16 (12.1%)48 (36.4%)68 (51.5%) |
| ER statusNegativePositive | 64 (48.5%)68 (51.5%) |
| PR statusNegativePositive | 67 (50.8%)65 (49.2%) |
NPI: Nottingham Prognostic Index; ER: estrogen receptor; PR; progesterone receptor.
This index has been validated as a measure of breast cancer prognosis and incorporates tumor grade, tumor size, and number of involved axillary lymph nodes. These three items are expressed as scores that are added together. Prognosis is favorable if NPI is ≤3.5, intermediate if >3.5 but <5.5 and poor if≥ 5.5. NPI values were calculated for 132 carcinomas.
Immunohistochemical stainingA streptavidin–biotin immunoperoxidase complex procedure was used for staining. Formalin-fixed, paraffin-embedded samples (4 μm thick) were deparaffinized and rehydrated, and endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 30 minutes. Antigen retrieval was carried out by microwave treatment in sodium citrate buffer (0.01 M, pH 6.0) for 10 minutes. Incubation with primary antibody was performed for E-cadherin (clone M3612, diluted 1:400; DAKO), N-cadherin (clone M3613, diluted 1:25; DAKO), Snail (clone CE2C3, diluted 1:100, Santa Cruz, CA, USA), estrogen receptor (ER) (clone 1D5, diluted 1:100; DAKO), and progesterone receptor (PR) (clone 636, diluted 1:100; DAKO) for 2 hours, followed by incubation with biotinylated secondary antibody for 30 minutes at room temperature. Slides were developed with diaminobenzidine (Sigma, USA). Finally, sections were counterstained with hematoxylin, dehydrated, cleared, and mounted. Each batch of staining included a negative control section treated with phosphate-buffered saline (PBS) instead of primary antibodies.
Immunohistochemical evaluationER and PR expression were scored from 0 to 2 as follows: 0 (negative): less than 5% of nuclei stained; 1 (borderline): 5–19% of nuclei stained; 2 (positive): more than 20% of nuclei stained.18
E-cadherin and N-cadherin expression were classified on the basis of the intensity of staining, the portion of the circumference of the cytoplasmic membrane stained and the percentage of cells exhibiting membranous staining. Grade 0: negative (no membranous staining identified); 1+: weak (faint staining involving a portion of the circumference of the cytoplasmic membrane in at least 10% of neoplastic cells); 2+: positive (moderate to definitive staining of the membrane over 100% of the cytoplasmic circumference in at least 10% of neoplastic cells); 3+: positive (strong positive staining of the membrane over 100% of the cytoplasmic circumference in at least 10% of neoplastic cells). For practical and statistical purposes, we grouped the weakly positive and negative cases together. Cases graded as moderate and strongly positive were considered positive tumors.19
Immunostaining with Snail produced cytoplasmic background staining in some cells, but only cells with nuclear protein accumulation indicative of transcriptional activity were considered positive. The sample was evaluated as positive, independently of intensity, if Snail was observed in >10% of nuclei. Cells that showed both nuclear and cytoplasmic staining were classified as positive for nuclear expression, in accordance with earlier studies.20
Statistical analysisStatistical analysis was performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). The chi-square test was performed to determine the correlations between clinicopathological parameters and protein expression. The Spearman's rho pairwise bivariate correlations test was used to estimate the relationships between the observed staining patterns. Results were considered significant at p≤0.05.
RESULTSPopulation and tumor characteristics
Patient age ranged from 39 to 71 years (mean 52.67, standard deviation 8.188). The clinicopathological characteristics of the patients are summarized in Table 1.
There was no significant correlation between the expression of any protein studied and age group or histological type (intraductal or invasive).
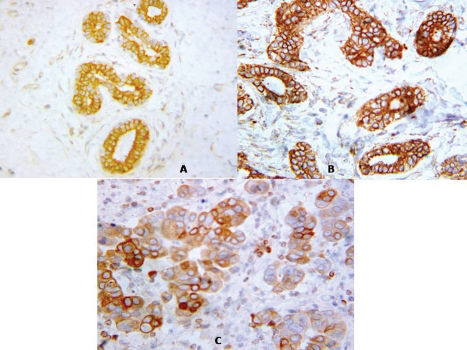
As shown in Figure 1, the intercellular borders of the luminal cells of normal glandular structures present in breast carcinoma specimens were strongly stained by E-cadherin. The myoepithelial cells of ducts and ductules showed much weaker or negative staining at cell–cell borders. Intraductal portions of IDCs showed strong staining for E-cadherin. A total of 37.1% of samples had lost E-cadherin expression (negative or weak). The absence of E-cadherin protein was significantly associated with all adverse clinicopathological variables. Negative E-cadherin expression was observed in 10% of grade I, 24.1% of grade II, and 53.1% of grade III tumors. There was a significant difference in E-cadherin down-regulation between grading groups (p = 0.001). A reduction in E-cadherin expression was detected in 49% of samples from the negative lymph node group and 80.9% of samples from the >3 positive lymph nodes group. There was a significant correlation between E-cadherin expression and lymph node group (p<0.001). Overall, 64.7% of the samples that were negative for E-cadherin immunoexpression were associated with poor NPI status (p<0.001). The absence of E-cadherin was also associated with positive expression of ER and PR (p<0.01 and 0.001 respectively) (Table 2).
(A) Immunohistochemical expression of E-cadherin was detected in normal breast glands. The luminal cells of the terminal ductules exhibit cytoplasmic membrane (linear intercellular) immunoreactivity, while no staining of the myoepithelial cells at the basal side of the epithelium was observed (X 400). (B) Immunohistochemical staining for E-cadherin in invasive ductal carcinomas of the breast. A well-differentiated tumor (grade I) showing strong cytoplasmic membrane staining localized almost exclusively to areas of cell–cell junctions is shown (X 400). (C) Poorly differentiated infiltrating ductal breast carcinomas (grade III) immunostained for E-cadherin, showing reduced and heterogeneous staining (X 400).
Expression of E-cadherin, N-cadherin and Snail in 132 invasive ductal breast carcinomas and their correlations with clinicopathological features.
| E-cadherin | N-cadherin | Snail | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Data | Negative (%) | P ositive (%) | p-value | Negative (%) | Positive (%) | p-value | Negative (%) | Positive (%) | p-value |
| n = 132 | 49 (37.1) | 83 (62.9) | 63 (47.7) | 69 (51.9) | 78 (59.1) | 54 (40.9) | |||
| Age<50 years≥50 years | 19 (36.5)30 (37.5) | 33 (63.5)50 (62.5) | 0.9 | 22 (42.3)41 (51.2) | 30 (51.2)39 (48.8) | 0.3 | 30 (57.7)48 (60) | 22 (42.6)32 (40) | 0.7 |
| Tumor size≤2 cm2-5 cm>5 cm | 1 (6.7)28 (34.1)20 (57.1) | 14 (93.3)54 (65.9)15 (42.9) | 0.002 | 11 (73.3)41 (50)11 (31.4) | 4 (26.7)41 (50)24 (68.6) | 0.02 | 14 (93.3)47 (57.3)17 (48.6) | 1 (6.7)35 (42.7)18 (51.4) | <0.01 |
| HistologyIDCIDC+DCIS | 30 (38.5)19 (35.2) | 48 (61.5)35 (64.8) | 0.7 | 35 (44.9)28 (51.9) | 43 (55.1)26 (48.1) | 0.4 | 48 (61.5)30 (55.6) | 30 (38.5)24 (44.4) | 0.4 |
| GradeGrade IGrade IIGrade III | 1 (10)14 (24.1)34 (53.1) | 9 (90)44 (39.7)30 (46.9) | 0.001 | 3 (30)38 (65.5)22 (34.4) | 7 (70)20 (34.5)42 (65.6) | 0.001 | 8 (80)42 (72.4)28 (43.8) | 2 (20)16 (27.6)36 (56.2) | 0.002 |
| LN statusNegative1-3 positive> 3 positive | 2 (49)9 (26.5)38 (80.9) | 49 (96.1)25 (73.5)9 (19.1) | <0.001 | 40 (78.4)13 (38.2)10 (21.3) | 11 (21.6)21 (61.8)37 (78.7) | <0.001 | 43 (84.3)19 (55.9)16 (34) | 8 (15.7)15 (44.1)31 (66) | <0.001 |
| StageIIIIII | 1 (7.7)10 (14.3)38 (77.6) | 12 (92.3)60 (85.7)11 (22.4) | <0.001 | 10 (76.9)46 (65.7)7 (14.3) | 3 (23.1)24 (34.3)42 (85.7) | <0.001 | 12 (92.3)50 (71.4)16 (32.7) | 1 (7.7)20 (28.6)33 (67.3) | <0.001 |
| NPIFavorableModeratePoor | 0 (0)5 (10.4)44 (64.7) | 16 (100)43 (89.6)24 (35.3) | <0.001 | 11 (68.8)35 (72.9)17 (25) | 5 (31.2)13 (27.1)51 (75) | <0.001 | 15 (93.8)37 (77.1)26 (38.2) | 1 (6.2)11 (22.9)42 (61.8) | <0.001 |
| ER statusNegativePositive | 36 (56.2)13 (19.1) | 28 (43.8)55 (80.9) | <0.001 | 22 (34.4)41 (60.3) | 42 (65.6)27 (39.7) | 0.001 | 24 (37.5)54 (79.4) | 40 (62.5)14 (20.6) | <0.001 |
| PR statusNegativePositive | 35 (52.2)14 (21.5) | 32 (47.8)51 (78.5) | 0.001 | 25 (37.3)38 (58.5) | 42 (62.7)27 (41.5) | 0.01 | 28 (41.8)50 (76.9) | 39 (58.2)15 (23.1) | 0.001 |
LN: lymph node; NPI, Nottingham Prognostic Index; ER: estrogen receptor; PR, progesterone receptor. Test of significance: chi-square test. P-values ≤0.05 are considered statistically significant.
As shown in Figure 2, normal breast ducts were negative for N-cadherin expression. Positive membranous expression (51.9% of cases) was associated with poor histologic differentiation. A significant correlation was found between N-cadherin expression and grading group (p = 0.001). N-cadherin expression was detected in 70% of grade I, 34.5% of grade II, and 65.6% of grade III samples. Similarly, 21.6% of samples from the negative lymph node group and 78.7% of samples from the >3 positive lymph nodes group were positive for N-cadherin expression (p<0.001). N-cadherin expression (75%) was associated with poor NPI status (p<0.001). A significant inverse association between membranous expression of N-cadherin and positive hormone receptor status was also detected (Table 2).
(A) N-cadherin overexpression in poorly differentiated invasive ductal carcinomas (grade III) showed positive cytoplasmic membrane staining for N-cadherin. Normal breast duct was negative for N-cadherin (upper right side) (X 400). (B) Immunohistochemical staining for N-cadherin in moderately differentiated invasive ductal carcinomas of the breast (grade II). Tumor cells show strong cytoplasmic membrane staining localized near cell–cell junctions (X 400). (C) Immunohistochemical staining for N-cadherin in invasive duct carcinomas of the breast. This poorly differentiated tumor (grade III) shows strong cytoplasmic membrane staining (X 400).
Snail (Figure 3): It was negative in normal glandular structures present within breast carcinoma. Snail was detected in 40.9% of total numbers of tumors. A significant correlation exists between Snail expression and tumor grade, with increased Snail expression observed with increasing tumor grade. More than half of grade III tumors (56.2%) expressed Snail, while 20% and 27.6% of grade I and II tumors exhibited nuclear Snail expression. Snail was frequently detected in node-positive tumors (44.1%) and less frequently detected in node-negative tumors (15.7%) (p<0.001). Snail expression (61.8%) was frequently observed in samples with poor NPI values (p<0.001). We also found a statistically significant correlation between Snail expression and negative ER and PR levels (Table 2).
(A) Immunohistochemical staining for Snail demonstrates strong nuclear localization in ductal carcinoma in situ of the breast (cribriform and solid types). The upper right side of the figure shows negative staining for Snail in normal human mammary gland lobules (X 200). (B) Immunohistochemical staining for Snail demonstrating nuclear localization in well-differentiated invasive ductal carcinomas of the breast (grade I). (X 400). (C) Immunohistochemical staining for Snail in invasive ductal carcinomas of the breast. This poorly differentiated tumor (grade III) exhibits strong nuclear staining (X 400).
Weak expression of membranous E-cadherin was strongly associated with the presence of membranous N-cadherin (87.8%) (p<0.001) and nuclear Snail (69.4%) (p<0.001). A significant association was observed between nuclear Snail expression and the absence of membranous E-cadherin (p<0.001; correlation coefficient -0.445). Positive associations were also observed between N-cadherin overexpression and the absence of membranous E-cadherin (p<0.001; correlation coefficient -0.546) and the presence of nuclear Snail (p<0.001; correlation coefficient -0.456) (Table 3).
Relationship among E-cadherin, N-cadherin and Snail expression in ductal breast carcinoma.
| N-cadherin | Snail | |||||
|---|---|---|---|---|---|---|
| Negative (%) | Positive (%) | p-value | Negative (%) | Positive (%) | p-value | |
| E-cadherin | ||||||
| Negative (%) | 6 (12.2) | 43 (87.8) | <0.001 | 15 (30.6) | 34 (69.4) | <0.001 |
| Positive (%) | 57 (68.7) | 26 (31.3) | 63 (75.9) | 20 (24.1) | ||
| Spearman correlation | -0.546 | <0.001 | -0.445 | <0.001 | ||
| N-cadherin | ||||||
| Negative (%) | 52 (82.5) | 11 (17.5) | <0.001 | |||
| Positive (%) | 26 (37.7) | 43 (62.3) | ||||
| Spearman correlation | -0.456 | <0.001 | ||||
Spearman's rho test. A two-sided P-value ≤0.05 was considered statistically significant.
Various signaling molecules and transcription factors regulate the expression of E-cadherin. Loss of E-cadherin induces epithelial–mesenchymal transition in several cancers, leading to increased tissue mobility and tumor invasion.21 In our study, E-cadherin was lost in 37.1% of tumors. Loss of E-cadherin–mediated cell adhesion is one of the key mechanisms involved in the metastatic conversion of epithelial cells and EMT.22,23 Partial or complete loss of E-cadherin is often correlated with an unfavorable prognosis, confirming that E-cadherin is important for maintaining the epithelial state.24,25 Furthermore, a significant correlation was found between the loss of E-cadherin and tumor grading group, lymph node group, and poor NPI status. The association of E-cadherin down-regulation with tumor grade has also been shown in other studies, although published results have been somewhat inconsistent;25,26 E-cadherin expression showed a significant relationship with lymph node metastases in one study,27 while it was independent of lymph node status in another.25 We also found associations between E-cadherin expression and ER and PR expression, in accordance with another study.25 ER-positive tumors express normal amounts of E-cadherin protein, and the loss of ER and E-cadherin genes has been linked to disease progression in invasive carcinomas of the breast. However, in another study, E-cadherin expression was not associated with a positive ER or PR status.28
In the present study, positive membranous expression of N-cadherin was detected in 51.9% of invasive ductal breast carcinoma samples and was associated with poor histologic differentiation. N-cadherin expression was increased in samples from patients with high tumor grade, poor NPI, positive lymph node infiltration and negative hormone receptors. High levels of N-cadherin expression are observed in most high-grade invasive ductal carcinomas, and N-cadherin is increased further in metastasizing subtypes, such as micropapillary breast cancers.12 This is in agreement with other studies, which have reported that N-cadherin expression is significantly higher in poorly differentiated carcinomas than in moderately or well-differentiated invasive ductal carcinomas.25,29 N-cadherin expression is detected in pre-invasive lesions, suggesting that increased N-cadherin expression may be involved in the transition from intraductal to invasive carcinoma. Thus, N-cadherin is associated with tumor aggressiveness and metastatic potential and may contribute to tumor progression.12,30
In our samples, Snail showed increased nuclear expression with increasing tumor grade. A relationship between Snail expression and cell differentiation has also been observed both in vitro and during early embryonic development.31,32 In addition, a correlation between Snail expression and lymph node status was detected in our study, i.e., Snail increased in node-positive tumors and decreased in node-negative tumors. This is in agreement with previous results, which showed that Snail expression correlates with the presence of lymph node metastases in IDCs. Snail was expressed in all IDCs with lymph node metastases, and all Snail-negative tumors were node-negative. Snail expression in epithelial cells induces not only profound changes in cell morphology but also the acquisition of tumorigenic and invasive properties. It has been concluded that Snail is involved in the progression of breast ductal carcinoma and may therefore serve as a marker of metastatic potential.33 We also found that Snail expression was significantly increased in samples with poor NPI status. In a recent study, no association was found between Snail and any poor prognostic factors.34 This contradicts previous findings that reported an association among Snail, poor prognostic factors and the presence of metastasis.32,33,35 Here, we found statistically significant correlations between Snail and ER and PR protein expression levels. Snail is a repressor that down-regulates the expression of aromatase (estrogen synthetase) in healthy breast tissue.33 It has been suggested that Snail may have a protective role against cancer in healthy breast tissue.36
Transcriptional silencing can cause reduced expression of E-cadherin.37 Interestingly, zinc finger transcription factors such as Snail are important for the epithelial–mesenchymal transition in embryonic development, during which E-cadherin expression is lost. Experimental knockdown of E-cadherin in epithelial breast cancer cell lines has confirmed that E-cadherin acts as a tumor suppressor.38,39 Our results reveal a significant association between the nuclear expression of Snail and the loss of membranous E-cadherin. Analyses of different mechanisms for E-cadherin inactivation in breast ductal carcinomas have shown that Snail is expressed in primary human tumors. A correlation between Snail expression and a reduction or lack of E-cadherin expression has been detected in a large number of tumors.32,33
Here, we have shown that N-cadherin expression is associated with a loss of membranous E-cadherin (correlation coefficient -0.546). Recent evidence indicates that N-cadherin is expressed in highly invasive tumor cell lines that lack E-cadherin expression. These findings raise the possibility that N-cadherin contributes to the invasive phenotype.40 Cadherin switching from E- to N-cadherin in epithelial malignancies has often been attributed to a switch from an epithelial to a mesenchymal phenotype,4 and this cadherin switching is an important event in cancer progression.41 Importantly, cadherin switching has an independent prognostic effect on time to biochemical failure and clinical recurrence in multivariate survival analyses, and this effect was stronger than that of either E-cadherin or N-cadherin alone.9 Consistent with our findings, a recent study of soluble E-cadherin and N-cadherin as serum biomarkers in prostate cancer showed that an increased level of N-cadherin is a marker of ongoing EMT and tumor progression.42,43
Down-regulation of E-cadherin and up-regulation of N-cadherin both occur at the transcriptional level. However, neither experimental knockdown nor experimental overexpression of N-cadherin interfered with morphological changes. In addition, the morphological changes associated with EMT preceded the down-regulation of E-cadherin.44 Up-regulation of N-cadherin in epithelial tumor cells contributes to the invasive/metastatic phenotype.12,45
In summary, our results indicate that N-cadherin is up-regulated in invasive ductal carcinoma of the breast. Loss of E-cadherin immunoexpression correlates significantly with tumor grade and other prognostic factors. N-cadherin expression in ductal breast carcinomas correlates with tumor aggressiveness, suggesting that cadherins could be useful for breast cancer diagnosis. Our present data strongly suggest the importance of EMT featuring increased N-cadherin and decreased E-cadherin expression in the progression and prognosis of patients with invasive ductal carcinoma. This switch is common and may be at least partly mediated by aberrant expression of Snail.
No potential conflict of interest was reported.