Currently, there are few centers able to perform pulmonary transplantation in Brazil.1 The number of patients in need of this procedure and their disease severity has increased in recent years; however, the reduced number of lung donors has resulted in a high mortality rate for patients on the waiting list.2 Some of these patients with exacerbations of severe chronic pulmonary disease can be temporarily supported by extracorporeal membrane oxygenation systems, prolonging life until a donor lung is available for transplantation.3 Those patients who are mechanically supported should receive the donated lungs with priority and should be shifted forward on the waiting list.4 In Brazil, there are no extracorporeal membrane oxygenation centers and, consequently, a prioritization system for patients awaiting lung transplantation using extracorporeal membrane oxygenation does not exist.5 This report describes our experience with an exacerbated severe hypoxemic patient awaiting lung transplantation and using extracorporeal membrane oxygenation in a tertiary center in Brazil.
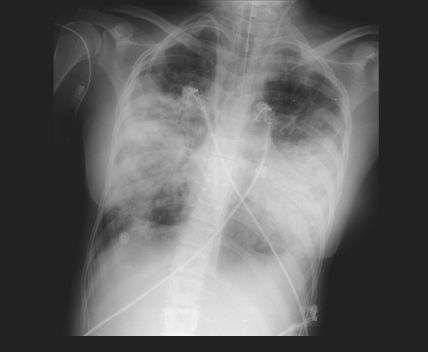
CASE REPORTAn eighteen-year-old woman was admitted to the respiratory intensive care unit of the Hospital das Clínicas de São Paulo, Brazil, with the diagnosis of pneumonia. The chest X-ray is shown in Figure 1. She had a previous history of advanced cystic fibrosis, was awaiting lung transplantation and was in the fifth position on the São Paulo State waiting list. During the first day of her respiratory intensive care unit stay, her status deteriorated, she was intubated, and mechanical ventilation was started. In spite of the ventilatory support, she developed severe hypoxemic and hypercapnic respiratory failure (Table 1), with no signs of hemodynamic compromise. Femoro-femoral venous-venous extracorporeal membrane oxygenation support was then initiated after ultrasound-guided placement of the cannulas. Arterial blood gases improved, promoting a comfortable spontaneous breathing pattern during mechanical ventilation. Trivial anticoagulation with heparin was started and titrated to a ratio of activated thromboplastin time of 1.5–2.3. The clinical characteristics during the patient's respiratory intensive care unit stay are shown in Table 1. The patient was maintained awake with a Richmond Agitation Sedation Scale score ranging from -1 to 0.
Clinical data and arterial blood gases.
| Data | Pre-ECMO | Day 1 | Day 2 | Day 3 | Day 4 | Day 18 |
|---|---|---|---|---|---|---|
| Mechanical ventilation | ||||||
| Ventilatory mode | VCV ∥ | PSV ∥ | PSV ∥ | PSV ∥ | PSV∥ | PSV∥ |
| Peak pressure (min – max) - cm H2O | 28 - 28 | 28 - 33 | 28 - 33 | 28 - 28 | 26 - 28 | 24 - 24 |
| PEEP (min – max) - cm H2O £ | 20 – 20 | 20 – 25 | 20 - 25 | 20 - 20 | 18 - 20 | 10 – 10 |
| FIO2 (min – max) ¥ | 0.6 – 0.7 | 0.7 – 0.7 | 0.6 – 0.7 | 0.6 – 0.6 | 0.6 – 0.6 | 0.6 – 0.6 |
| Respiratory rate (min – max) - breaths/min | 22 - 30 | 25 - 36 | 18 - 34 | 22 - 39 | 24 – 36 | 22 - 38 |
| ECMO€ | ||||||
| Blood flow (min – max) - L/min | 6.0 – 6.5 | 6.0 – 6.0 | 5.5 – 6.0 | 5.0 – 5.5 | 5.0 – 5.0 | 5.0 – 5.0 |
| Sweeper flow (min – max) - L/min | 6.0 – 10.0 | 6.0 – 6.0 | 4.0 – 6.0 | 2.5 – 4.0 | 2.5 – 2.5 | 2.5 – 2.5 |
| FIO2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Routine blood gas | ||||||
| PaO2 - mm Hg | 42 | 50 | 62 | 74 | 55 | 81 |
| PaCO2 - mm Hg | 118 | 51 | 38 | 53 | 51 | 51 |
| SBE - mEq/L ∗ | 1.8 | 6.1 | -1.0 | 0.9 | -0.8 | 2.1 |
| pH | 7.10 | 7.41 | 7.43 | 7.33 | 7.32 | 7.36 |
| Patient data | ||||||
| RASS (min – max)# | -1 - 0 | 0 - 0 | 0 – 0 | 0 – 0 | 0 – 0 | -5 - 0 |
| Lung injury score¶ | 4.00 | 4.00 | 4.00 | 3.75 | 3.75 | 3.00 |
| Total SOFA score § | 10 | 9 | 6 | 4 | 4 | 4 |
| Respiratory SOFA | 4 | 4 | 4 | 4 | 4 | 4 |
| Cardiovascular SOFA | 3 | 3 | 0 | 0 | 0 | 0 |
| Hematological SOFA | 1 | 1 | 1 | 0 | 0 | 0 |
| Hepatic SOFA | 1 | 0 | 0 | 0 | 0 | 0 |
| Neurological SOFA | 0 | 0 | 0 | 0 | 0 | 0 |
| Renal SOFA | 1 | 1 | 1 | 0 | 0 | 0 |
SOFA denotes sequential organ failure assessment. This is a score to diagnose and quantify organ failure, which ranges from 0 to 24.
The patient's condition gradually improved, and daily weaning from extracorporeal membrane oxygenation was performed by zeroing the sweeper flow of the oxygenation membrane. Our criteria for extracorporeal membrane oxygenation removal are as follows: (1) the patient is awake and comfortable throughout the test and (2) PaO2 ≥55 mmHg and PaCO2 ≤60 mmHg (or pH≥7.30 in patients with chronic hypercapnia) after one hour of ventilation with PEEP ≤10 cm H2O, FIO2 ≤0.6 and a tidal volume ≤6 mL/kg (or a driving pressure ≤12 cm H2O). We abort the test when (1) the peripheral oxygen saturation is less than 85%, (2) the patient presents clinical signs of dyspnea, or (3) the staff deems such an action to be necessary. The patient remained on extracorporeal membrane oxygenation support for 18 days with no adverse events, but she never tolerated more than five minutes of the weaning test. After 18 days of extracorporeal membrane oxygenation support, the patient died.
DISCUSSIONIn the case presented, before the initiation of extracorporeal membrane oxygenation support, we promoted an extensive discussion between the respiratory intensive care unit and transplantation teams in order to define the focus of care. The background of the discussion was the absence, in Brazil, of prioritization criteria for patients on the waiting list for lung transplantation who require extracorporeal membrane oxygenation support when weaning from extracorporeal membrane oxygenation is considered difficult or impossible. The final decision was to start extracorporeal membrane oxygenation support and to file a special request to the Ministry of Health, asking to prioritize the patient on the lung transplantation waiting list. Our expectation was that with full intensive care support and antibiotics, the patient would gradually improve toward a clinical condition that was sufficient to allow lung transplantation. In the meantime, our request was analyzed by the Ministry of Health.
The extrapulmonary organ dysfunctions of the patient quickly resolved; from a clinical standpoint, she was able to undergo lung transplantation from the third day of her stay in the respiratory intensive care unit. Despite her clinical improvement, she remained completely dependent on extracorporeal membrane oxygenation support (i.e., the use of extracorporeal membrane oxygenation as a bridge to lung transplantation). On the 17th day of her stay in the respiratory intensive care unit, the Ministry of Health approved prioritization of the patient on the lung transplantation list on an exceptional basis due to the circumstances. During this period, no transplant had been performed from donors with the same blood type as the patient. Unfortunately, the patient died on the 18th day of her stay in the respiratory intensive care unit.
In summary, we believe that our experience with this case should motivate revision of the current legislation regulating lung transplants in Brazil, as well as the procurement, conservation, and reconditioning of organ systems. The scenario in which patients with exacerbations of chronic severe pulmonary diseases will become dependent on the support of extracorporeal devices will become frequent. Should the clinical conditions allow, prioritization on the waiting list for lung transplantation for these patients should be carefully considered.
The ECMO group comprises: Luciano Cesar Pontes Azevedo, Marcelo Park, André Luiz de Oliveira Martins, Eduardo Leite Vieira Costa, Guilherme Paula Pinto Schettino, Marcelo Brito Passos Amato, Carlos Roberto Ribeiro Carvalho, Mauro Tucci, Alexandre Toledo Maciel, Fernanda Maria Queiroz Silva, Leandro Utino Taniguchi, Edzângela Vasconcelos and Adriana Sayuri Hirota.