Popliteal artery aneurysms are frequent and may lead to thromboembolic events and limb loss.
PURPOSETo evaluate clinical and ultrasonographic follow-up of patients who underwent exclusion of a popliteal artery aneurysm using the technique proposed by Edwards.
METHODSData of all patients who underwent surgery to repair a popliteal artery aneurysm at Hospital das Clinicas, the São Paulo University Medical School between 1996 and 2004 were reviewed. Inclusion criteria were repair with aneurysm exclusion and bypass using the technique proposed by Edwards, as well as the existence of preoperative and postoperative measurements of the aneurysmal sac.
RESULTSData of 16 patients who underwent 20 procedures for popliteal artery aneurysm exclusion and bypass were available to analysis. The preoperative diameter of the popliteal artery aneurysms ranged from 1.3 cm to 6.1 cm (mean = 3.1 cm). Patients underwent duplex ultrasound scanning 1 month to 7 years after surgical repair. Follow-up of the 20 cases revealed that 10 aneurysms exhibited decreased mean transverse diameters, ranging from 0.2 to 2.3 cm, while 7 had increased in diameter, ranging 0.3 to 3.3 cm, and 3 remained unchanged. Flow was observed only in 5 outo f the 20 procedures, 3 of which (60%) had increased diameters.
CONCLUSIONAlthough exclusion is a widely accepted procedure for the repair of popliteal artery aneurysms, data in the literature and the results of this study, which did not include cases of rupture or compression, suggest that strict follow-up of patients who undergo aneurysm exclusion is necessary.
Os aneurismas da artéria poplítea são freqüentes e estão associados a eventos trombo-embólicos que podem acarretar isquemia grave com risco de perda da extremidade inferior acometida.
OBJETIVOAvaliar a evolução clínica dos pacientes e ultra-sonográfica dos aneurismas de artéria poplítea excluídos pela técnica de Edwards.
MÉTODOAnálise retrospectiva dos pacientes com diagnóstico de aneurisma da artéria poplítea operados no Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, no período compreendido entre os anos de 1996 a 2004. Foram incluídos os pacientes submetidos à exclusão do aneurisma pela técnica de Edwards e que possuíam diâmetro transverso máximo do aneurisma mensurado no período pré e pós-operatório.
RESULTADOSDezesseis pacientes foram submetidos a correção cirúrgica de 20 aneurismas. O diâmetro do aneurisma no período pré-operatório variou entre 1,3 cm a 6,1 cm (média 3,1cm). O controle ultrasonográfico foi realizado em intervalo de 1 mês a 7 anos do procedimento cirúrgico. Houve diminuição do diâmetro do aneurisma de artéria poplítea em 10/20 extremidades (variação de 0,2 cm a 2,3 cm), aumento em 7/20 (variação de 0,3 cm a 3,3 cm) e estabilidade em 3/20. Observou-se a ocorrência de fluxo no saco aneurismático em 5 dentre os 20 procedimentos. Destes, três apresentaram crescimento do mesmo (60% dos casos com fluxo).
CONCLUSÃOEsta amostra de pacientes, sem rotura ou sinais e sintomas de compressão, associada à análise da literatura, demonstra que o seguimento estreito do aneurisma excluído é necessário.
Popliteal artery aneurysms are frequent and may lead to thromboembolic events and limb loss.1–4, as is the case with other limb arterial pathologies.5,6
In 1969, Edwards described a technique for exclusion of the aneurysmal sac by means of proximal and distal ligation and autogenous vein interposition graft.7 This technique avoids dissection of the aneurysm and reduces edema of the limb and the risk of vein or nerve lesion. However, collateral branches may be left without ligation, which may result in persistent perfusion of the excluded aneurysm. This prevents complete sac thrombosis and eventually leads to sac enlargement.
The purpose of this study was to use ultrasonographic analysis, a frequently employed tool,8 in conjunction with a clinical evaluation for the follow-up of patients who underwent exclusion of popliteal artery aneurysms using the technique proposed by Edwards.
METHODSData of all patients who underwent surgery to treat popliteal artery aneurysm (PAA) at HCFMUSP between 1996 and 2004 were reviewed. All patients underwent clinical follow-up and duplex ultrasound surveillance.
Patients included in the study had aneurysms repaired by exclusion and bypass using the technique proposed by Edwards.
Analysis excluded patients without preoperative ultrasound measurement of the aneurysmal sac and patients for whom postoperative measurement was impossible due to death, amputation, or loss to follow-up.
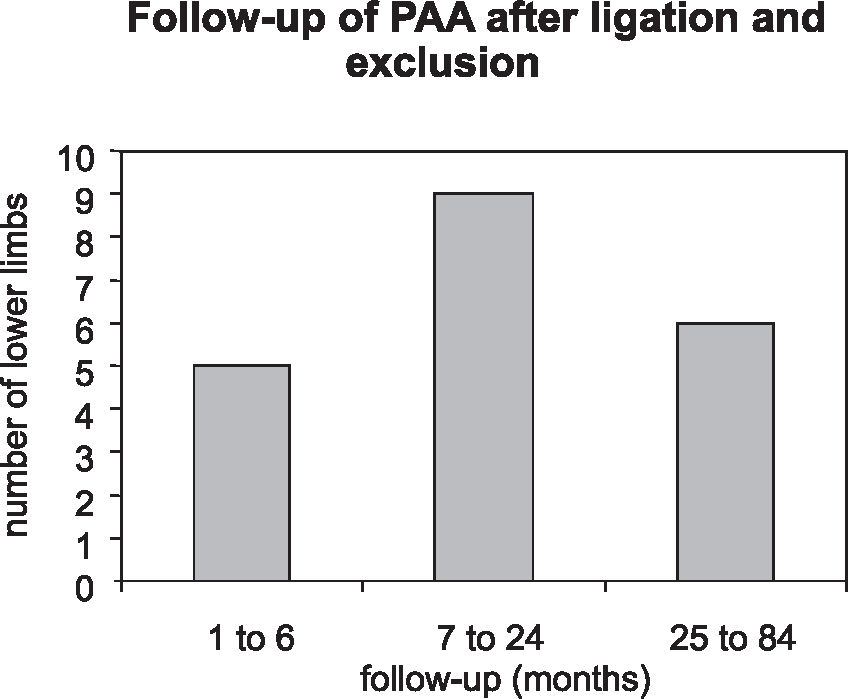
Patients returning for routine clinical follow-up were examined for signs and symptoms of compression developed after surgery: pain, paresthesia, paresis, or venous hypertension. Duplex scanning was used to measure the greatest transverse diameter of the excluded aneurysm. Color Doppler imaging was used to evaluate intrasac flow. The interval from surgical repair to control duplex scanning was variable, as shown in Figure 1.
Twenty-seven patients underwent 34 primary popliteal aneurysm repair procedures. Preoperative measurements of the PAA were available for only 24 cases. Two patients had died, 1 had had a limb amputation, and 5 were lost to follow-up. Therefore, data of 16 patients and 20 PAA exclusion and bypass procedures were available for analysis. Table 1 shows patient characteristics and their aneurysms in this study.
Patient characteristics and associated aneurysms in this study
| N | % | |
|---|---|---|
| Patients | 16 | |
| Male | 14 | 87.5 |
| Age at diagnosis | ||
| Range | 43 to 86 years | |
| mean | 68.7 years | |
| Comorbidities | ||
| Hypertension | 13 | 81.3 |
| Diabetes mellitus | 2 | 12.5 |
| Hyperlipidemia | 5 | 31.2 |
| Active smoker | 3 | 18.8 |
| Ex-smoker | 11 | 68.8 |
| CAD | 2 | 12.5 |
| Associated aneurysms | ||
| abdominal aorta | 5 | 31.2 |
| femoral artery | 1 | 6.3 |
| contralateral PAA | 7 | 43.8 |
| subclavian artery | 1 | 6.3 |
Observations: 7 patients had contralateral popliteal artery aneurysms (PAA), but 3 of them did not have preoperative measurements of popliteal diameter. Therefore, only 4 patients had the 2 lower limbs included in the study.
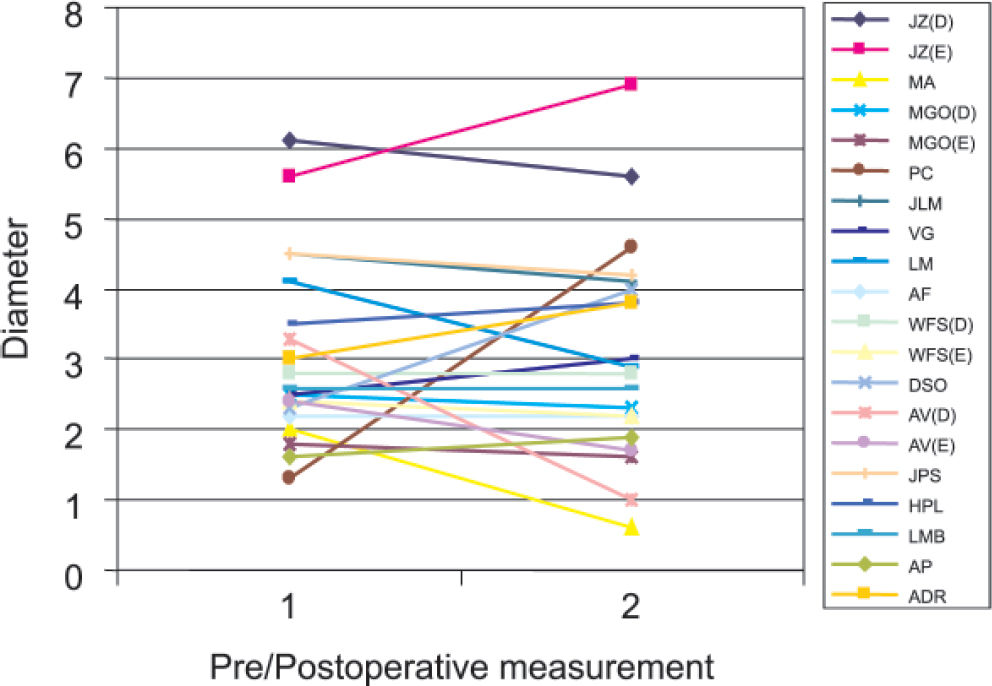
Preoperative PAA diameters ranged from 1.3 cm to 6.1 cm (mean = 3.1 cm). Duplex ultrasound scanning was performed 1 month to 7 years after surgical repair (median = 11.5 months). The follow-up of patients revealed that of the 20 PAA, 10 had a decrease of 0.2 to 2.3 cm in mean transverse diameter, 7 had an increase of 0.3 cm to 3.3 cm, and 3 remained unchanged. Duplex ultrasound did not detect any intrasac flow in 15 PAA. However, in 5 cases flow was detected, 3 (60%) of which exhibited increased diameters (Table 2). One patient with an enlarged aneurysm underwent limb amputation because of acute graft thrombosis. No other patient developed any symptoms, and the management adopted was duplex ultrasound scanning every 6 months or earlier if clinical changes were detected. One of these asymptomatic patients had a marked increase in diameter of 3 cm, but this patient refused to undergo clinical and ultrasonographic evaluation and could only be contacted by phone.
Diameter variation and intrasac flow detection by duplex scanning
| Name | Preoperative diameter (cm) | Postoperative diameter (cm) | Variation | Flow | Length of postoperative follow-up (months) |
|---|---|---|---|---|---|
| JZ | R: 6.1 | 5.6 | −0.5 | Yes | 9 |
| L: 5.6 | 6.9 | 1.3 | Yes | 9 | |
| MA | 2.0 | 0.6 | −1.4 | No | 17 |
| MGO | R: 2.5 | 2.3 | −0.2 | No | 5 |
| L: 1.8 | 1.6 | −0.2 | No | 5 | |
| PC | 1.3 | 4.6 | 3.3 | No | 3 |
| JLM | 4.5 | 4.1 | −0.4 | No | 8 |
| VG | 2.5 | 3.0 | 0.5 | No | 17 |
| LM | 4.1 | 2.9 | −1.2 | No | 18 |
| AF | 2.2 | 2.2 | 0 | No | 36 |
| WFS | R: 2.8 | 2.8 | 0 | No | 13 |
| L: 2.4 | 2.2 | −0.2 | No | 30 | |
| DSO | 2.3 | 4.0 | 1.7 | No | 33 |
| AV | R: 3.3 | 1.0 | −2.3 | No | 84 |
| L: 2.4 | 1.7 | −0.7 | No | 84 | |
| JPS | 4.5 | 4.2 | −0.3 | Yes | 9 |
| HPL | 3.5 | 3.8 | 0.3 | No | 11 |
| LMB | 2.6 | 2.6 | 0 | No | 1 |
| AP | 1.6 | 1.9 | 0.3 | Yes | 12 |
| ADR | 3.0 | 3.8 | 0.8 | Yes | 1 |
R = right lower limb; L = left lower limb.
The risk of thromboembolic events associated with PAA ranges from 18% to 31%,2,4,9–12 whereas rupture is found in only 2% to 3% of most large series.10 Surgical treatment is the most effective method for preventing complications. However, no consensus about indications for surgery has been reached. Most authors suggest surgery for symptomatic patients with intermittent claudication, digital atheroembolism, rupture, critical ischemia by acute aneurysm thrombosis, or in cases of vein or nerve compression, or for asymptomatic patients with transverse diameters greater than 2.0 cm. Intramural thrombi are associated with a higher risk of embolization and therefore are an indication for surgical repair.2,3,9–11,13
Surgical repair techniques have been described since ancient Greece. Antilllus, in 200 BC, reported an aneurysm ligation followed by packing of the sac. In 1785, Hunter, in England, and Desaut, in France, carried out a superficial femoral artery ligation in the adductor canal, the so-called Hunterian ligation. Matas, in 1885, developed the endoaneurysmorrhaphy technique, which lowered the limb amputation rate from 10.5% to 5.2%.14–16 In 1969, Edwards7 proposed a surgical technique with minor trauma, and this has become the most common method of repair since then.3 The technique is an alternative to extensive exposure through the section of the tendons of the semimembranosus, gracilis, semitendinosus and sartorius muscles, and medial head of the gastrocnemius muscle, steps that are essential in the medial exposure of the popliteal artery, opening of the aneurysmal sac, ligation of collateral branches, and graft interposition.
The posterior approach, another alternative technique,15,16 is recommended for aneurysms confined to the popliteal fossa and that do not reach the Hunter hiatus superiorly. The aneurysm can be repaired by opening the sac and ligating collateral branches, avoiding the complications associated with the exclusion technique without resection of tendons. Endovascular repair has been recently described with immediate success but having high occlusion rates. Careful long-term follow-up and larger series should be studied to determine the durability of endoluminal stent grafts in comparison with traditional bypass surgery before its wide-scale use is recommended.17,18
Few studies report on PAA outcomes after exclusion and revascularization of the limb. Jones et al19 found that proximal and distal ligation adjacent to the PAA is more effective in isolating the aneurysm than long segment isolation, probably because of the large number of collateral branches filling the aneurysmal sac. They also observed that single proximal or distal ligation resulted in significant aneurysm growth.19 Ebaugh et al17 reviewed 25 cases of PAA treated with ligation and exclusion bypass grafting and reported 8 cases of enlargement of the postoperative diameter. They attributed these results to a phenomenon similar to type II endoleak or endotension after aortic endovascular repair due to flow from genicular branches. Kirkpatrick et al20 studied patients with popliteal aneurysms who underwent ligation and bypass procedures and showed that one third had persistent flow in the aneurysm sac on late postoperative Doppler ultrasound scanning, and attributed this flow to patent genicular branches. Mehta et al21 and Battey22 reported finding intrasac flow and cases of rupture in excluded PAA, and they suggested opening of the aneurysmal sac and ligation of visible branches as the best technique for surgical repair. Flynn et al23 and Cinelli et al24 reported unusual complications of bypassed politeal aneurysms.
In this study, 7 of the 20 surgically repaired PAA (35%) exhibited an increase in the transverse diameter. Intrasac flow may be explained by loose proximal or distal ligation or by reperfusion by collaterals. Growth without flow might have occurred because of pressurization of the excluded PAA by collaterals, or because of ligation failure. Table 3 compares our results with those reported by other authors, confirming that reperfusion of the aneurysmal sac does occur and should be a matter of concern. Patients in this study were all asymptomatic during follow-up.
Comparison of patients that underwent ligation and exclusion of the aneurysmal sac
| Author | No. Patients | No. PAA | Male | Age (range) | Increase in Diameter | Unchanged diameter | Reduction in diameter | Follow-up length (range) |
|---|---|---|---|---|---|---|---|---|
| Jones | 30 | 41 | 29 | 76 years(67.3-84.7) | 12* (33%) | 5 | 24 | 46 months (4-88 m) |
| Ebaugh | 18 | 25 | 17 | 63 years (42-80) | 8(32%) | 5(20%) | 12(48%) | |
| Kirkpatrick | 25 | 72 years(47-86) | 13 | 6 | 6 | 59 months(1-120 m) | ||
| Flow- | 13 | 5 (38%) | 5 | 3 | ||||
| Flow+ | 12 | 8** | 13 | |||||
| Wakassa | 16 | 20 | 14 | 68.7 years(43-86) | 7 | 3 | 10 | 11 months(1-84 m) |
| Flow- | 15 | 4 (27%) | 3 | 8 | ||||
| Flow+ | 5 | 3*** | 02 |
Although the treatment of PAA by exclusion is widely accepted, the results found for this series of patients without rupture or compression, as well as results reported in the literature, show that patients who undergo aneurysm exclusion should be strictly followed up.