Genomic instability is a hallmark of malignant tissues. In this work, we aimed to characterize nuclear and mitochondrial instabilities by determining short tandem repeats and somatic mitochondrial mutations, respectively, in a cohort of Brazilian sporadic breast cancer cases. Furthermore, we performed an association analysis of the molecular findings and the clinical pathological data.
METHODS:We analyzed 64 matched pairs of breast cancer and adjacent non-cancerous breast samples by genotyping 13 nuclear short tandem repeat loci (namely, D2S123, TPOX, D3S1358, D3S1611, FGA, D7S820, TH01, D13S317, D13S790, D16S539, D17S796, intron 12 BRCA1 and intron 1 TP53) that were amplified with the fluorescent AmpFlSTR Identifiler Genotyping system (Applied Biosystems, USA) and by silver nitrate staining following 6% denaturing polyacrylamide gel electrophoresis. Somatic mtDNA mutations in the D-loop site were assessed with direct sequencing of the hypervariable HVI and HVII mitochondrial regions.
RESULTS:Half of the cancer tissues presented some nuclear instability. Interestingly, the D13S790 locus was the most frequently affected (36%), while the D2S123 locus presented no alterations. Forty-two percent of the cases showed somatic mitochondrial mutations, the majority at region 303-315 poly-C. We identified associations between Elston grade III, instabilities at 13q31 region (p = 0.0264) and mtDNA mutations (p = 0.0041). Furthermore, instabilities at 13q31 region were also associated with TP53 mutations in the invasive ductal carcinoma cases (p = 0.0207).
CONCLUSION:Instabilities at 13q31 region and the presence of somatic mtDNA mutations in a D-loop site correlated with tumor aggressiveness.
Breast cancer is the most prevalent cancer that affects women worldwide. One of the most striking characteristics of this disease is the heterogeneity of its genetic and pathological aspects (1). Genomic instability is one of the hallmarks of cancerous tissues, and it increases in advanced and more aggressive tumors (2,3). This instability may involve large chromosomal alterations, such as chromosomal deletions or duplications, and lead to allelic loss or amplification. In addition to the epigenetic mechanisms, the loss of heterozygosity (LOH), which results in allelic imbalance, is a common method of hampering tumor suppressor gene activities during carcinogenesis. TP53 and RB are good examples of tumor suppressor genes that are frequently altered by allelic imbalance (3). Short tandem repeats (STRs) or microsatellites are polymorphic regions that are widely used to analyze allelic imbalance in tumors. In breast cancer, LOH has been detected at several loci in both familial and sporadic breast cancers, with frequencies ranging between 20% and 79% (4,5). Recently, Tokunaga et al. (6) studied the microsatellite instability of five randomly selected loci in Japanese primary breast cancer samples. They observed that a high frequency of LOH was associated with triple-negative and high-grade HER2 breast cancers. When the same research group specifically evaluated microsatellite instability at the BRCA1 locus, they demonstrated that LOH at this region was independently associated with disease-free survival (7). In addition to nuclear genomic instabilities, researchers have also considered mitochondrial genomic alterations as indicators of cell commitment to carcinogenesis. Although their involvement is currently not well understood, somatic mitochondrial DNA (mtDNA) mutations seem to participate in cancer development in different ways (8,9). Lim et al. (10) demonstrated that mtDNA mutations in colorectal cancer might be implicated in risk factors that induce poor outcomes and tumorigenesis. Tseng et al. (11) suggested that somatic mtDNA mutations may play a critical role in breast cancer progression.
The aim of this study was to characterize nuclear instabilities and mitochondrial genomic mutations in a cohort of Brazilian sporadic breast cancer cases. We analyzed matched pairs of breast cancer and adjacent non-cancerous breast samples by genotyping 13 nuclear STR loci [namely, D2S123, TPOX, D3S1358, D3S1611, FGA, D7S820, TH01, D13S317, D13S790, D16S539, D17S796, intron 12 BRCA1 and intron 1 TP53] and by directly sequencing HVI and HVII mitochondrial regions. Furthermore, we performed an association analysis of the molecular findings and clinical pathological data from the cases.
PATIENTS AND METHODSTumor samplesTissue specimens from sporadic primary breast cancer tumors and the corresponding adjacent tumor-free areas were obtained between 2005 and 2009 from the biopsies of 64 women at the Fernandes Figueira Institute, FIOCRUZ, Rio de Janeiro, Brazil. After excision, the tissues were snap-frozen in liquid nitrogen and stored at -70oC. Cancer diagnosis was confirmed by histopathology. Sixty-four percent of cases were diagnosed as invasive ductal carcinoma, and 36% were classified as invasive lobular carcinoma, mucinous, or micropapillary. DNA was extracted from the tissue samples using a salting-out method (12). The DNA was quantified using ethidium bromide staining in agarose gels and UV spectrophotometry at 260 nm. The P53 and estrogen/progesterone receptor levels, which were assessed by immunohistochemistry, and the clinical-pathological data were obtained from records of the department of pathology, IFF-FIOCRUZ. The study protocol was approved by the local ethics committee.
mtDNA sequencingHypervariable mitochondrial DNA regions I and II (D-loop region) were sequenced using the dideoxy chain termination method (BigDye® Terminator v3.1 Cycle Sequencing Kit) and analyzed in an automated ABI310 Sequencer (Applied Biosystems, USA). All of the sequences were aligned to the Revised Cambridge Reference Sequence, accession number NC_012920. The primer pairs designed for the PCR and direct sequencing of mtDNAs are provided in Supplementary Table 1. The mitochondrial somatic mutation data were assessed by comparing cancerous and adjacent non-cancerous breast samples.
Clinical-pathological aspects of the cases and an association analysis of STR instabilities and mtDNA mutations (n = 64).
| Clinical-pathological | All STR instabilities | Instability at 13q31 ¤¤ | Somatic mtDNA mutations §) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aspects | n | S (n = 31) | U (n = 33) | p-value | S (n = 37) | U (n = 27) | p-value | WT (n = 37) | M (n = 27) | p-value |
| Age (years) | ||||||||||
| <55 | 37 | 19 | 18 | 21 | 16 | 22 | 15 | |||
| ≥55 | 27 | 12 | 15 | 0.6210 | 16 | 11 | 1.0000 | 15 | 12 | 0.8017 |
| Ethnic group | ||||||||||
| African | 27 | 12 | 15 | 16 | 11 | 18 | 9 | |||
| Non-African | 37 | 19 | 18 | 0.6210 | 21 | 16 | 1.0000 | 19 | 18 | 0.3063 |
| European | 26 | 12 | 14 | 13 | 13 | 15 | 11 | |||
| Non-European | 38 | 19 | 19 | 0.8035 | 24 | 14 | 0.3161 | 22 | 16 | 1.0000 |
| AA | 11 | 7 | 4 | 8 | 3 | 4 | 7 | |||
| Non-AA | 53 | 24 | 29 | 0.3312 | 29 | 24 | 0.3311 | 33 | 20 | 0.1792 |
| Tumor size | ||||||||||
| ≤2 cm (T1) | 31 | 17 | 14 | 19 | 12 | 18 | 13 | |||
| >2 cm (T2+T3) | 28 | 11 | 17 | 0.2994 | 16 | 12 | 0.7952 | 10 | 18 | 0.1188 |
| Lymph node¤ | ||||||||||
| Negative | 33 | 15 | 18 | 18 | 15 | 17 | 16 | |||
| Positive | 26 | 13 | 13 | 0.7900 | 16 | 10 | 0.7900 | 11 | 15 | 0.6013 |
| Histological subtype | ||||||||||
| IDC | 44 | 21 | 23 | 26 | 18 | 26 | 18 | |||
| Others | 20 | 10 | 10 | 1.0000 | 11 | 9 | 0.7904 | 11 | 9 | 0.7904 |
| Elston grade (n = 53) | ||||||||||
| I+II | 40 | 22 | 18 | 27 | 13 | 25 | 15 | |||
| III | 13 | 4 | 9 | 0.2021 | 4 | 9 | 0.0264∗) | 2 | 11 | 0.0041∗∗) |
| Progesterone receptor | ||||||||||
| Positive | 32 | 12 | 20 | 18 | 14 | 19 | 13 | |||
| Negative | 31 | 19 | 12 | 0.0793 | 19 | 12 | 0.7994 | 11 | 20 | 0.0787 |
| Estrogen receptor | ||||||||||
| Positive | 47 | 24 | 23 | 30 | 17 | 29 | 18 | |||
| Negative | 16 | 7 | 9 | 0.7735 | 7 | 9 | 0.2397 | 1 | 15 | 0.0001∗∗) |
| p53 | ||||||||||
| Positive | 19 | 7 | 12 | 9 | 10 | 7 | 12 | |||
| Negative | 44 | 24 | 20 | 0.2737 | 28 | 16 | 0.2721 | 23 | 21 | 0.2866 |
| TP53 mutation | ||||||||||
| WT | 50 | 27 | 23 | 32 | 18 | 33 | 17 | |||
| Mutant | 14 | 4 | 10 | 0.1322 | 5 | 9 | 0.0724 | 4 | 10 | 0.0162∗) |
¤¤ 13q31 region: D13S317 and D13S790 STR loci.
n - Total number of samples; S - Number of stable samples; U - Number of unstable samples; AA - Asian-Amerindian; mtDNA – Mitochondrial DNA.
WT - Wild type; M – Mutation; IDC - Invasive Ductal Carcinoma.
¤ Lymph node metastasis: Negative (N0); Positive (N1+N2+N3).
Nuclear genomic instability was assessed by PCR analysis of 13 STR markers. The TPOX, D3S1358, FGA, D7S820, TH01, D13S317 and D16S539 loci were amplified with the fluorescent AmpFlSTR Identifiler Genotyping system according to the manufacturer's recommendations (Applied Biosystems, USA) and then analyzed using the automated ABI3100 Genetic Analyzer platform and GeneMapper Software (Applied Biosystem, USA). The D13S790 locus was amplified with an independent FAM-fluorescent system and analyzed using the ABI3100 Genetic Analyzer platform (Applied Biosystems, USA). The D2S123, D3S1611, D17S796, intron 12 BRCA1 and intron 1 TP53 loci were analyzed using silver nitrate staining following a 6% denaturing polyacrylamide gel electrophoresis. Nuclear genome instability was assessed by observing the allelic imbalances, which are usually identified as LOH. Supplementary Table 1 shows the STR loci localizations and the primer sequences. When the allelic patterns differed between the matched normal and tumor DNAs, the PCRs and electrophoresis were performed twice. Eventually, the lymphocyte DNAs of patients were also genotyped and compared to normal and tumor DNAs to confirm results. In a previous study, TP53 mutation detection was performed for exons 4-9 (13). The association analyses were performed with Fisher's exact test with a significance level of 95% using GraphPad® software.
RESULTSClinical-pathological aspects of casesTo obtain all the possible noteworthy clinical-pathological data from the studied cases, the 64 patients were evaluated for age, ethnicity, histological classification, TNM, Elston grade, p53 and estrogen and progesterone receptor expression levels (Table 1) and Supplementary Tables 2 and 3). The average age of the studied patients was 53, and the ages ranged from 27 to 76 years. The ethnic classification was based on mitochondrial haplogroups. The patients were classified into three ethnic groups: African (42%), European (40%) and Asian-Amerindians (18%). Most of the cases (69%) were diagnosed as invasive ductal carcinomas (IDCs). The other histological subtypes, which represented a total of 18 cases (31%), included the following subtypes: invasive papillary carcinoma, comedocarcinoma, mucinous and medullar intraductal carcinoma. Most of the cases (75%) were classified at low or intermediate grades, although 25% were Elston grade III (high aggressiveness). Fifty percent of the cases were progesterone-positive, and 74% were estrogen-positive. In relation to the p53 tumor suppressor protein, 70% of the cases were protein-negative, and 22% were mutant (13).
Association analysis of TP53 and mtDNA mutations with STR instabilities in invasive ductal carcinoma cases (n = 44).
| Clinical-pathological | All STR instabilities | Instability at 13q31¤ | |||||
|---|---|---|---|---|---|---|---|
| aspects | n | S (n = 23) | U (n = 21) | p-value | S (n = 26) | U (n = 18) | p-value |
| TP53 mutation | 14 | ||||||
| WT | 35 | 21 | 24 | 11 | |||
| Mutant | 9 | 2 | 7 | 0.0642 | 2 | 7 | 0.0207∗) |
| mtDNA mutations§) | |||||||
| WT | 26 | 15 | 11 | 17 | 9 | ||
| Mutant | 18 | 8 | 10 | 0.5406 | 9 | 9 | 0.3613 |
¤13q31region: D13S317 and D13S790 STR loci.
n – Total number of samples; S - Number of stable samples; U - Number of unstable samples; WT - Wild Type.
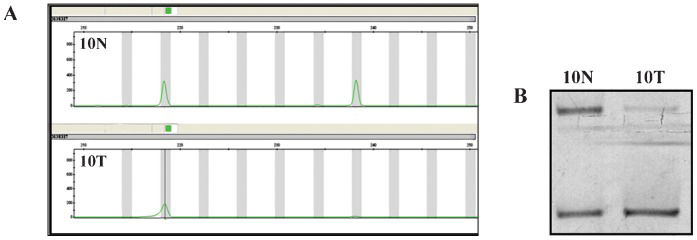
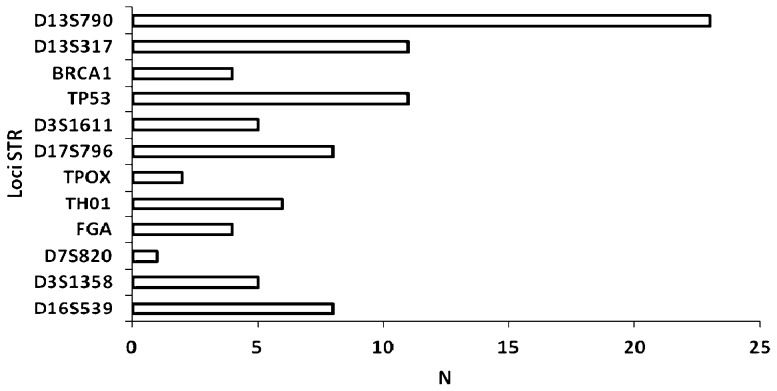
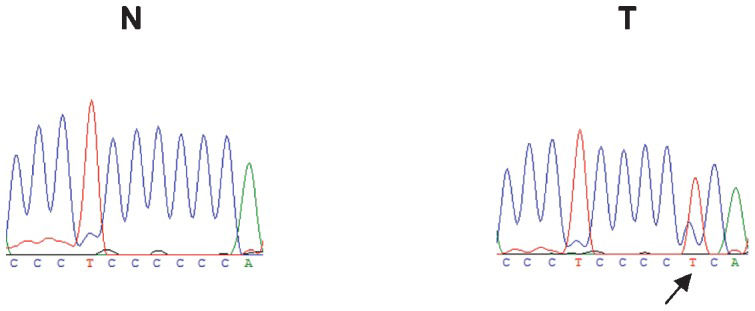
To investigate the genomic instability of our breast cancer cases, both the nuclear and mitochondrial DNAs were analyzed. Nuclear genome instabilities were detected by analyzing the forensic CODIS-recommended STR loci (i.e., D2S123, TPOX, D3S1358, FGA, D7S820, TH01, D13S317, D16S539) and the STRs that were designed for this study (i.e., D3S1611, D13S790, D17S796, intron 12 BRCA1 and intron 1 TP53). Figure 1 shows an example of LOH detection at the D13S317 locus using the fluorescent Identifiler system and a silver-stained polyacrylamide gel. Approximately half of the cases displayed microsatellite instability to some extent; this instability was characterized by allelic imbalances and 41% of cases exhibited alterations in three or more loci. Among the 13 analyzed STR loci, only the D2S123 locus was stable and the D7S820 locus had the lowest frequency of instability (1%). The intron 1 TP53 and D13S317 loci were each unstable in 16% of cases. Interestingly, the D13S790 locus had the highest frequency of instability among the STR loci (36%). Figure 2 displays the distribution of the number of instabilities in the STR loci. Supplementary Table 4 summarizes the data that was obtained from each of the 64 cases. Regarding the mitochondrial genome analysis, 42.18% of cases had somatic mutations, most of which were at the 303-315 poly-C region (Supplementary Table 4). Figure 3 illustrates an example of mtDNA mutation assessed by direct sequencing.
Following the determination of nuclear instabilities and mitochondrial genomic alterations, an association study with clinical-pathological aspects was performed. Interestingly, when the most frequent unstable genome region (13q31, assessed here through the microsatellite markers D13S317 and D13S790) was analyzed separately, it was statistically associated with Elston grade III (p = 0.0264) (Table 1). Furthermore, a positive association was also observed with the presence of TP53 mutations in IDCs (p = 0.0207) (Table 2). A highly positive association with Elston grade III was also observed with the presence of somatic mtDNA mutations (p = 0.0041). Moreover, reinforcing their correlation with parameters of tumor aggressiveness, the mtDNA mutations were statistically associated with negative estrogen receptor expression (p = 0.0001) and TP53 mutations (p = 0.0162). There was no correlation between the STR instabilities and the somatic mtDNA mutations.
DISCUSSIONSeveral molecular mechanisms are involved in the formation and progression of breast carcinomas, particularly sporadic breast cancers. An important feature of breast tumor development is the characteristic but highly heterogeneous genomic instability (14). Recently, the advantageous utilization of genome-scale analysis and microarray-based gene expression profiling has stressed the complexity of breast cancer progression (15,16). This study was designed and executed to provide further understanding of genomic instability in Brazilian breast cancer cases. We performed nuclear STR loci genotyping and direct sequencing of HVI and HVII mitochondrial regions of 64 matched pairs of cancerous and adjacent non-cancerous breast samples. Our main aims were to detect genomic instabilities in well-known DNA regions using selected STR loci and the mitochondrial D-loop region and to analyze their association with clinical aspects. With the results, we could expect to have a clearer understanding of local and defined genomic changes, both nuclear and mitochondrial, and their clinical consequences. Surprisingly, through the microsatellite markers D13S317 and D13S790, we found that 13q31 was the most frequent unstable genomic region. It was most apparent at the D13S790 locus, with more than 20 cases presenting LOH. When analyzed separately from the other chromosomal loci, 13q31 was shown to be statistically associated with Elston grade III in all breast tumors and with TP53 mutations in invasive ductal carcinomas, both of which are clinical parameters of tumor aggressiveness (17,18). The 13q31 locus has been described as a chromosome region that shows different genetic alterations depending on the cancer type. Genetic gains have been observed in sarcoma (19) and colorectal cancer (20). Genetic losses have also been verified in breast cancer (21,22). Eiriksdottir et al. (23) analyzed chromosome 13q in detail in 139 sporadic breast tumors with 18 polymorphic microsatellite markers and identified 3 LOH target regions: 13q12-q13, 13q14 and 13q31-q34. In another study, correlations were detected between the allelic loss of the D13S1694 marker (telomeric to BRCA2) and both larger tumor sizes and negative estrogen receptors (24). More recently, Schwarzenbach et al. (25), studying cell-free DNA in benign and malignant breast tumor cases, noted that LOH at D13S280 and D13S159, both markers located at 13q31-33, are associated with overall and disease-free survival. In this same study, all of the analyzed markers significantly correlated with lymph node status (25). Together, these results and our results suggest the existence of a putative suppressor gene or an important regulator sequence in this region. The miR17-92 cluster (13q31.3 region) is located near the 13q31 region; the cluster consists of seven microRNAs tightly grouped within an 800 bp genomic region in the third intron of the primary transcript C13orf25. This cluster is also known as oncomir-1 because its superexpression has been demonstrated in pulmonary cancer and lymphomas (26,27). However, there is some evidence of LOH in this genomic region, mainly in breast cancer, indicating that this cluster can also play a role as a tumor suppressor gene (28,29). Our results reinforce the hypothesis that instability in the 13q31 region may relate to a loss of function of microRNAs in this cluster. Because most of the allelic imbalances were associated with Elston grade III, and (more importantly) 13q31 LOH was associated with TP53 mutations in the IDC samples, we can infer that this alteration is a delayed event in breast tumor progression. We also investigated somatic mutations in the D-loop region of the mtDNA and found that 42.18% of cases were mutated, the majority at the 303-315 poly-C region. As has been described by others (30,31), we could demonstrate an association between the presence of mtDNA mutations and breast tumor aggressiveness. Parameters such as high histological grade (Elston grade III), estrogen receptor-negative and TP53 mutations were statistically associated. Kuo et al. (32) recently reported that the presence of somatic mutations in the D-loop indicates poor prognosis; however, they did not identify a correlation with the presence of TP53 mutations in 30 pairs of tumor and non-tumor samples. The low number of samples and/or the different types of breast cancer cases could explain the difference. TP53 and somatic mtDNA mutations have been considered to be good biomarkers of nuclear DNA damage (18,32); therefore, a correlation between both genetic alterations would be expected. However, we did not identify any association between nuclear instabilities and mtDNA alterations. Alazzouzi et al. (33) also observed that mitochondrial alterations were not associated with nuclear instability in breast tumors. In a study of colorectal carcinomas, instability in the 303 poly-C region of mtDNA was not associated with nuclear microsatellite instability (34). These observations suggest an independent occurrence of both phenomena. In conclusion, although the number of the Brazilian cases evaluated in this study was not high, we could highlight an important role for instabilities at the nuclear 13q31 locus and in mtDNA in breast cancer development and prognosis.
The authors thank the patients for their collaborative participation in this study. Gilson Costa dos Santos Junior and Humberto de Vitto were recipients of fellowships from CNPq/Brazil, and Carla Cristina Moreira was a recipient of a fellowship from PIBIC/CNPq/Brazil. We also thank Angela Duarte, Genomic Platform, UERJ, for her technical assistance. This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Nuclear STR and mtDNA primer sequences.
| Locus | Chromosomelocalization | Motif | Primer sequences | Amplicon(bp) | |
|---|---|---|---|---|---|
| TPOX | 2p23 | AATG | ACTGGCACAGAACAGGCACTTAGGGGAGGAACTGGGAACCACAGAGGTTA | FR | 224-252 |
| D2S123 | 2p16(hMSH2) | CA | AAACAGGATGCCTGCCTTTAGGACTTTCCACCTATGGGAC | FR | 197-227 |
| D3S1611 | 3p21(hMLH1) | CA | CCCCAAGGCTGCACTTAGCTGAGACTACAGGCATTTG | FR | 260-268 |
| D3S1358 | 3p21 | TCTA | ACTCGAGTCCAATCTGGTTATGAAATCAACAGAGGCTTG | FR | 97-147 |
| FGA | 4p28 | TTTC | GCCCCATAGGTTTTGAACTCATGATTTGTCTGTAATTGCCAGC | FR | 206-332 |
| D7S820 | 7q11 | GATA | GATTCCACATTTATCCTCATTGACATGTTGGTCAGGCTGACTATG | FR | 215-247 |
| TH01 | 11p15 | AATG | ATTCAAAGGGTATCTGGGCTCTGGGTGGGCTGAAAAGCTCCCGATTAT | FR | 179-203 |
| D13S790 | 13q31 | GATA | TTGAGCCAGGATGATGTGCCTTTGGGTTGTAAACGT | FR | 422-454 |
| D13S317 | 13q31 | TATC | ACAGAAGTCTGGGATGTGGAGCCCAAAAAGACAGACAGAA | FR | 165-197 |
| D16S539 | 16q24 | GATA | GGGGGTCTAAGAGCTTGTAAAAAGGTTTGTGTGTGCATCTGTAAGCAT | FR | 264-288 |
| BRCA1 | 17q(intron 12 BRCA) | TG | GGTCATGTGTTCCATTTGGGTTGAAGCAACTTTGCAATGAG | FR | 190-270 |
| D17S796 | 17p | CA | CAATGGAACCAAATGTGGTCAGTCCGATAATGCCAGGATG | FR | 144-174 |
| TP53 | 17p(intron 1 TP53) | AAAAT | GCACTGACAAAACATCCCCTAGTAAGCGGAGATAGTGCCACTGT | FR | 150-180 |
| HVI | mtDNA | - | CGCACCTACGTTCAATATTACAGGGGTGTGTGTGTGCTGGGTAGG | FR | 364 |
| HVII | mtDNA | - | ATTACTGCCAGCCACCATGAAACGTGTGGGCTATTTAGGCTTTA | FR | 445 |
F-Forward; R-Reverse.
Clinical-pathological patient data.
| Case | Age(Years) | Ethnicity§) | Histologicalclassification | TNM | EG | ImmunohistochemistryPR ER P53 | ||
|---|---|---|---|---|---|---|---|---|
| T2 | 52 | African | IDC | pT1c pN0 (sn) pMx | I | +++ | +++ | - |
| T4 | 48 | African | IDC | pT1c pN0 (sn) pMx | II | + | + | - |
| T5 | 53 | African | IDC | pTis pN0 (sn) pMx | ∗) | ND | ND | ND |
| T6 | 56 | European | IDC | pT2c pN2a pMx | III | - | - | + |
| T8 | 49 | African | IDC | pT1c pN0 (sn) pMx | I | +++ | +++ | - |
| T9 | 60 | African | Invasive lobular | pT2c pN0 (sn) pMx | ∗) | - | +++ | - |
| T10 | 44 | AA | IDC | pT2 pN0 pMx | III | - | - | + |
| T11 | 27 | African | Intracystic papillary | pTis pN0 pMx | ∗) | + | +++ | - |
| T14 | 54 | African | IDC | pT2 pN2a pMX | II | + | +++ | - |
| T15 | 41 | African | IDC | pT1c pN0 (sn) pMx | I | - | +++ | + |
| T16 | 48 | AA | IDC | pT1c pN0 (sn) pMX | I | - | +++ | - |
| T17 | 46 | European | IDC | pT2 pN1a pMx | II | - | - | - |
| T18 | 54 | European | IDC | pT1c pN2a pMX | ∗) | +++ | +++ | - |
| T19 | 50 | African | Mucinous | pT1c pN0 (sn) pMX | I | - | +++ | - |
| T21 | 39 | AA | IDC | pT1b pN0 pMX | III | - | - | + |
| T23 | 55 | African | IDC | pT2 pN1a pMx | I | +++ | +++ | - |
| T25 | 46 | European | IDC | pT1c pN0 pMX | II | - | +++ | - |
| T26 | 60 | African | IDC | pT3 pN0 pMx | III | - | - | - |
| T27 | 72 | African | Invasive papillary | pT1c pNx pMx | II | - | + | - |
| T28 | 46 | European | IDC | pT1c pN0 (sn) pMx | III | - | +++ | - |
| T29 | 70 | African | Invasive papillary | pT2 pN0 (sn) pMX | I | ++ | +++ | - |
| T31 | 36 | African | Invasive micropapillary | pT2 pN1a pMx | III | - | - | - |
| T32 | 50 | AA | IDC | pT1c pN0 pMx | I | - | ++ | - |
| T33 | 56 | European | IDC | pT1c pN2a pMx | III | - | +++ | - |
| T34 | 46 | European | IDC | pT2 pN1a pMx | III | - | - | + |
| T35 | 49 | European | IDC | pT1c pN0 (sn) pMx | II | +++ | - | - |
| T36 | 53 | European | IDC | pT2 pN0 (sn) pMx | II | - | - | + |
| T37 | 47 | European | Mucinous | pT1c pN0 (sn) pMx | I | - | + | - |
| T38 | 61 | African | IDC | pT1b pN0 (sn) pMx | I | +++ | +++ | - |
| T40 | 66 | African | IDC | pT1c pN2 pMx | III | - | +++ | + |
| T42 | 40 | African | IDC | pT2 pN0 (sn) pMx | I | +++ | +++ | - |
| T43 | 52 | AA | IDC | pTis pN0 (sn) pMx | ∗) | - | - | - |
| T44 | 58 | African | IDC | pT2 pN1a pMx | II | +++ | +++ | - |
| T46 | 44 | European | IDC | pT2 pN3a pMx | II | - | +++ | - |
| T47 | 71 | European | IDC | pT2 pN0 pMx | II | +++ | +++ | + |
| T48 | 40 | European | IDC | pT1c pN0 pMx | II | - | - | + |
| T50 | 42 | African | Invasive lobular | pT1a pN1a pMx | ∗) | ++ | +++ | + |
| T52 | 40 | European | IDC | pT2 pN1a pMx | II | ++ | +++ | - |
| T53 | 60 | European | IDC | pT1c pN1a pMx | I | - | +++ | - |
| T55 | 40 | European | Invasive apocrine | pT1a pN1a pMx | ∗) | + | + | + |
| T56 | 74 | AA | IDC | pT2 pN1a pMx | II | - | +++ | - |
| T58 | 70 | AA | Invasive lobular | pT2 pN1a pMx | ∗) | +++ | +++ | - |
| T59 | 46 | African | Invasive apocrine | pT2 pN1a pMx | II | - | - | + |
| T60 | 58 | European | IDC | pT2 pN1a pMx | I | ++ | +++ | - |
| T61 | 44 | AA | IDC | pT2 pN1b1 pMx | II | + | + | + |
| T62 | 76 | European | IDC | pT1 pN0 pMx | II | + | + | - |
| T63 | 71 | African | IDC | pT1 pN1 pMx | I | + | + | - |
| T65 | 53 | African | Invasive papillary | ND | II | - | + | - |
| T68 | 59 | African | Invasive micropapillary | pT2 pN3 pMx | III | - | - | + |
| T69 | 72 | European | Invasive lobular | pT1 pN0 pMx | ∗) | + | + | + |
| T70 | 50 | European | IDC | pT2 pN0 pMx | II | + | + | - |
| T71 | 63 | European | Invasive lobular | pT1 pN1 pMx | ∗) | + | + | + |
| T72 | 68 | European | IDC | pT2 pN0 pMx | III | - | - | + |
| T73 | 63 | African | Invasive papillary | pT1 pN2 pMx | III | - | - | - |
| T74 | 75 | European | IDC | pT1 pN0 pMx | III | - | - | + |
| T75 | 41 | European | IDC | pT2 pN1 pMx | II | + | + | - |
| T76 | 60 | AA | Invasive micropapillary | pT2 pN2 pMx | II | + | + | - |
| T77 | 46 | African | IDC | pT1 pN0 pMx | I | + | + | - |
| T78 | 66 | European | IDC | pT1 pNx pMx | II | + | + | - |
| T80 | 28 | AA | IDC | pTis pN0 pMx | ∗) | - | + | + |
| T81 | 47 | African | IDC | pT2 pN0 pMx | II | + | + | - |
| T82 | 69 | European | Invasive micropapillary | pT1 pNx pMx | II | + | + | - |
| T83 | 49 | African | Invasive mixed type | pT2 pNx pMx | II | + | + | - |
| T85 | 61 | AA | Invasive apocrine | pT1 pN0 pMx | II | + | + | - |
IDC – Invasive ductal carcinoma; TNM – Tumor-lymph node metastasis; EG – Elston grade; PR – Progesterone receptor; ER – Estrogen receptor; Protein expression: (-) negative, (+) positive - 25-50%, (++) positive - 50-75%; (+++) positive - more than 75%; ND - no data; AA - Asian-Amerindian.
Classification of cases according to the clinical-pathological aspects (total = 64).
| Variables | Number of samplesn (%) |
|---|---|
| Age (years) | |
| <4545-5555-6565-75>75 | 14 (22)24 (37)13 (20)12 (19)1 (2) |
| Tumor size | |
| T1 (≤2 cm)T2 (> 2 cm)T3 (> 5 cm)Tis (Carcinoma in situ)ND | 31 (48)27 (42)1 (2)4 (6)1 (2) |
| Lymph node metastasis | |
| N0N1N2N3NxND | 33 (52)17 (26)7 (11)2 (3)4 (6)1 (2) |
| Histological subtype | |
| IDCInvasive LobularOthers § | 44 (69)5 (8)15 (23) |
| Elston grade∗ | |
| IIIIII | 15 (28)25 (47)13 (25) |
| Progesterone receptor | |
| PR +PR ++PR +++PR –ND | 18 (28)4 (6)10 (16)31 (48)1 (2) |
| Estrogen receptor | |
| ER +ER ++ER +++ER –ND | 20 (31)1 (2)26 (40)16 (25)1 (2) |
| P53 | |
| p53+p53-ND | 19 (30)44 (68)1 (2) |
IDC - Invasive ductal carcinoma; § Other histological subtypes - Invasive papillary, comedocarcinoma, mucinous, medullar intraductal; PR - Progesterone receptor; ER - Estrogen receptor; High levels of protein expression - +: 25-50%, ++: 50-75%, +++:>75%; -: Normal levels or low levels of protein expression; ∗ Elston grade was applied only for the IDC subtype and other types of IDC; ND – Not detected.
Unstable STR loci, mtDNA mutations and TP53 mutation status (exons 4-9).
| Case | Unstable STR loci | Mitochondrial somatic mutations | TP53 mutation |
|---|---|---|---|
| T2 | D17S796,D13S790 | - | G245S |
| T4 | D13S790 | - | - |
| T5 | D13S790 | - | - |
| T6 | - | 303-315C (8-9) TC (6) | - |
| T8 | - | 16192 CC/T | - |
| T9 | D13S790 | 16309 AA/G | - |
| T10 | D3S1358, D13S317, D17S796, D3S1611, BRCA1 | 303-315 C (7-8) TC (6) | R248Q |
| T11 | D13S790 | - | - |
| T14 | TH01, TP53, D3S1611, D3S1358, D17S796 | 303-315 C (7-8) TC (6) | - |
| T15 | - | - | - |
| T16 | - | 303-315 C (7-8) TC (6)16391 GG/A | - |
| T17 | - | 303-315 C (7-8) T C (6)16261 CC/T | - |
| T18 | - | - | R175H |
| T19 | - | 303-315 C (7-8) TC (6) | H168P |
| T21 | FGA, D3S1358, D3S1611, D13S790 | 303-315 C (8-9) TC (6) | R273H |
| T23 | - | - | - |
| T25 | - | 16192CC/T | - |
| T26 | TP53,FGA, D16S539, D13S317 | - | - |
| T27 | - | - | - |
| T28 | - | - | - |
| T29 | D16S539, D17S796 | 146 TT/C | - |
| T31 | FGA, D13S317, TH01, BRCA1, D13S790 | - | 16888delC |
| T32 | - | - | - |
| T33 | D13S790 | - | 16897-16911del |
| T34 | D13S790 | 303-315 C (8-9) TC (6)66 GG/T | Y234C |
| T35 | D13S790, D17S796, TP53 | - | - |
| T36 | - | - | - |
| T37 | D13S317 | - | - |
| T38 | TP53 | - | |
| T40 | D13S317, FGA, TH01, D17S796, D3S1611, TP53, BRCA1, D3S1358, TPOX,D13S790 | 294 TT/C | I195L |
| T42 | - | 16261CC/T | - |
| T43 | - | 303-315 C (7-8) TC (6) | - |
| T44 | - | - | - |
| T46 | - | - | - |
| T47 | D16S539, TP53, TPOX | - | - |
| T48 | - | - | - |
| T50 | TP53 | - | - |
| T52 | TP53,D13S317, D16S539,D13S790 | - | - |
| T53 | - | - | - |
| T55 | - | 294 TT/C | W146stop |
| T56 | - | - | - |
| T58 | - | - | - |
| T59 | TP53,TH01, BRCA1, D3S1358, D16S539, D13S317, D13S790 | 303-315 C (7-8) TC (6)338 CC/T | P278A |
| T60 | TH01,D16S539, D13S317 | - | - |
| T61 | TP53,D13S317,D13S790 | 215 AA/G | - |
| T62 | - | 303-315 C (8-9) TC (6) | - |
| T63 | - | - | - |
| T65 | - | - | - |
| T68 | - | - | - |
| T69 | D13S790 | 303-315 C (7-8) TC (6)338 CC/T | - |
| T70 | D13S790 | - | - |
| T71 | D13S790 | 215AA/C | - |
| T72 | D7S820,TH01, D16S539, D17S796,D13S790 | 303-315 C (7-8) TC (6) | R175H |
| T73 | - | - | - |
| T74 | D13S790 | 303-315 C (7-8) TC (6)16291 CC/T | - |
| T75 | - | - | - |
| T76 | D3S1611 | 303-315 C (7-8) TC | D259V |
| T77 | - | - | - |
| T78 | D13S790 | 303-315 C (7-8) TC (6)16291CC/T | - |
| T80 | - | - | - |
| T81 | D13S790,D13S317 | 303-315 C (7-8) TC (6) | - |
| T82 | D13S790,D16S539 | - | - |
| T83 | D13S790 | - | - |
| T85 | - | - | - |
No potential conflict of interest was reported.
Santos-Jr GC was responsible for the STR genotyping, patient data collection, statistical analysis and critical revision of the paper. Goes AC was responsible for the STR genotyping study design and execution and critical review of the manuscript. De Vitto H was responsible for mutant mtDNA design, execution and results interpretation. Moreira CC performed STR genotyping. Avad E was responsible for the patient samples and data collection. Rumjanek FD was responsible for partial financial support. De Moura Gallo CV conceived and designed the study, was responsible for research support and manuscript writing.