Investigation of resuscitation fluids in our swine hemorrhage model revealed moderate to severe chronic pneumonia in five swine at necropsy. Our veterinary staff suggested that we perform a retrospective analysis of prospectively collected data from these animals. We compared the data to that of ten healthy swine to determine the physiologic consequences of the added stress on our hemorrhage/resuscitation model.
METHODS:Anesthetized, immature female swine (40 ± 5 kg) were instrumented for determining arterial and venous pressures, cardiac output and urine production. A controlled hemorrhage of 20 ml/kg over 4 min 40 sec was followed at 30 min by a second hemorrhage of 8 ml/kg and resuscitation with 1.5 ml/kg/min of LR solutions to achieve and maintain systolic blood pressure at 80 ± 5 mmHg for 3.5 hrs. Chemistries and arterial and venous blood gasses were determined from periodic blood samples along with hemodynamic variables.
RESULTS:There were significant decreases in survival, urine output, cardiac output and oxygen delivery at 60 min and O2 consumption at 120 min in the pneumonia group compared to the non-pneumonia group. There were no differences in other metabolic or hemodynamic data between the groups.
CONCLUSION:Although pneumonia had little influence on pulmonary gas exchange, it influenced cardiac output, urine output and survival compared to healthy swine, suggesting a decrease in the physiologic reserve. These data may be relevant to patients with subclinical infection who are stressed by hemorrhage and may explain in part why some similarly injured patients require more resuscitation efforts than others.
Uncontrolled hemorrhage is a potentially serious consequence of traumatic injury on the battlefield and in the civilian environment and is a major cause of morbidity and mortality.1-3 During severe hemorrhage (50 - 60% blood loss) and subsequent shock resulting from the reduced blood flow and oxygen delivery, there is a characteristic shift toward metabolic acidosis,4 with an increase in plasma lactate, and a decrease in plasma bicarbonate and blood buffers, including protein. The outcome can become fatal very quickly if aggressive resuscitation measures are not taken. Fluid resuscitation to extend survival has been the standard for many years. However, over the past decade, hypotensive resuscitation or a permissive hypotension has been investigated and recommended.5-8
Improved and novel approaches to fluid resuscitation to improve blood pressure and tissue perfusion and preservation are under investigation. In an investigation of the potential benefits of select adjuncts added to lactated Ringer's solution for resuscitation of swine following hemorrhage, it was noted that some of the swine responded differently to hemorrhage and died earlier than the majority. At necropsy, these swine were found to have pre-existing but asymptomatic moderate to severe pneumonia.
Pneumonia is the inflammation and consolidation of lung parenchyma caused by a variety of bacterial pathogens, such as Pneumococcus, Staphylococcus, Pseudomonas, Klebsiella, Legionella, Mycoplasma, but they can also include viral and fungal pathogens, as well as chemical agents (smoke and chlorine) and trauma.9 Many pneumonia-causing pathogens are opportunistic and they are usually present in the nasopharynx.9,10
Pneumonia is common in man, and mycoplasmal pneumonia is the most prevalent disease in swine, with a high morbidity (30–80%) and low mortality.11 Most mycoplasmal pneumonias in swine become complicated by secondary bacterial or viral infections; however, some uncomplicated lesions may resolve spontaneously. Clinical symptoms such as coughing, sneezing, fever or decreased appetite may not be present.12 Therefore, mycoplasmal pneumonia in experimental swine can be present without the knowledge of the investigator or the supporting veterinary staff. Pneumonia is often not diagnosed until necropsy.
Due to its size and many similarities to man, swine has become an important animal model for hemorrhagic shock research.13-15 Discarding data after determination of preexisting pathology (pneumonia) in the experimental subject can be very expensive and disruptive. Even the purchase of SPF animals, with assurance of a healthy animal rather than a less expensive farm animal, may not guarantee that the animal is free from underlying asymptomatic pathology, such as pneumonia.
Upon the suggestions of our veterinary staff, this retrospective analysis of prospectively collected data was initiated to determine the influence that asymptomatic pneumonia has on the outcome of swine hemorrhage and resuscitation in our model.
MATERIALS AND METHODSThe original investigation was approved by the Institutional Animal Care and Use Committee of the U.S. Army Institute of Surgical Research, Fort Sam Houston, TX. All animals received care in strict compliance with the 1996 Guide for the Care and Use of Laboratory Animals by the National Research Council and were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility. This retrospective analysis was performed on data obtained from a previously approved investigation – no additional animals were used. The analysis included five animals with chronic pneumonia and ten healthy swine.
Animal PreparationImmature female Yorkshire swine weighing 40 ± 5 kg were obtained from a local USDA Class A vendor (John Albert Yorkshire Farm, Cibolo, TX), quarantined for one week and screened for any blood abnormalities. It should be noted that male domestic swine are normally castrated at one week of age, and that female swine do not become sexually mature until six months of age.16 The swine in this study were 3.5 ± 0.1 [range 2.7–5.1] months old, such that no effect of gender on the response to the injury used in this model is expected to be present. Previous studies performed with swine of this age of both sexes showed no differences on any parameters due to gender, although a Grade V liver injury model was used instead of a controlled hemorrhage.17 Before the investigation, the animals were fasted overnight with unlimited access to water. On the morning of the study, the animals were premedicated with 1 cc/18 kg of Glycopyrrolate, IM, to control salivation, and sedated with Telezol (3 mg/kg), IM, followed by anesthesia with isoflurane (2-3%) in 50% O2 using an Ohmeda 7800 ventilator at a tidal volume of 11 ml/kg. An end-tidal PCO2 of 40 ± 2 mmHg during the baseline period was achieved and monitored using an Ohmeda 5250 RGM (BOC Healthcare, Madison, WI). Swine core temperature was maintained at 37-39 °C.
Two ventral, longitudinal neck incisions were made for exposure and isolation of the left and right external jugular veins and carotid arteries. A 0.05-inch OD polyvinyl chloride catheter was placed in a carotid artery and advanced into the thoracic aorta to monitor arterial blood pressure and heart rate. A 7F Swan-Ganz thermodilution catheter (American Edwards Laboratory, Inc., Irvine, CA) was inserted into a jugular vein and the tip was advanced to a wedge position in the pulmonary artery. The catheter was then withdrawn slightly for measurement of the pulmonary artery pressure. The Swan-Ganz catheter was also used to measure right ventricular pressure, central venous pressure and cardiac output (CO). Continuous CO was measured by a 16-mm flow probe placed around the ascending aorta (Transonic Systems, Inc., Ithaca, NY). It should be noted that the incision to place the flow probe was not sufficiently large to fully inspect the lungs, which would have permitted identification of the pneumonia prior to the experiment. Teflon Angiocath catheters (18 ga, 4.25 in; Arrow International, Reading, PA) were inserted non-occlusively into the opposite jugular vein and carotid artery and a Paratrend catheter (PT7, Diametrics Medical, Inc., Roseville, MN) was inserted through each Angiocath for continuous blood gas measurement. Another incision was made for exposure and isolation of the femoral vein and artery. An 8.5F sheath introducer catheter (American Edwards Laboratory, Inc., Irvine, CA) was inserted into the femoral vein for fluid resuscitation and the femoral artery for blood sampling and hemorrhage. Muscle oxygenation was assessed non-invasively using a near infrared probe (INVOS 4100, Somanetics Corp., Troy, MI) on the medial surface of the thigh and the rib cage. ECG leads were placed on the animal's limbs and chest.
A midline ventral laparotomy was performed and the spleen was removed and weighed. The animal was then infused with warm lactated Ringer's solution at three times the spleen weight to replace the splenic blood volume. Abdominal organs were inspected for evidence of past or present disease. After instrumentation and surgical manipulation, isoflurane was reduced to 1.2% and ketamine (Fort Dodge Animal Health, Fort Dodge, IA; 100 µg/kg/min) was infused into an ear vein to maintain anesthesia. During hemorrhage and resuscitation, the isoflurane dose was adjusted, as needed, to maintain surgical anesthesia.
Systolic, diastolic and mean arterial blood pressures, pulmonary artery pressure, right ventricular pressure, central venous pressure, CO, heart rate ECG, and ventilator parameters (tidal volume, inspired and expired airway pressures) from the Ohmeda RGM anesthesia monitor were continuously monitored. All analog and digital data were acquired and stored on a Dynamic Recording Evaluation Workstation (DREW, U.S. Army Institute of Surgical Research, San Antonio, TX).
After animal stabilization, 10-min baseline data collection was started. Arterial and venous blood samples were taken at baseline, at the blood pressure nadir, and at 15, 30, 60, 90, 120, 180 and 240 min after initiation of the first hemorrhage. The blood was analyzed for 1) arterial and venous blood gases, pH and base excess (AVL Omni 9 Analyzer, Roche Diagnostics, Indianapolis, IN); 2) hematocrit (Hct), hemoglobin (Hb) and complete blood count (Pentra-120 Hematology Analyzer, ABX Diagnostics, Irvine, CA); 3) total plasma protein, glucose, creatinine, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase (CK), amylase and lactate (Vitros Chemistry System, Ortho Clinical Diagnostics, Raritan, NJ); and 4) baseline prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen and platelet concentration (ACL Futura Automatic Coagulation Analyzer, Instrumentation Laboratories, Lexington, MA). INR was determined by dividing each individual PT value by the average of the baseline PT measurements from all animals in the study (avg = 10.6 ± 0.1 sec; n = 91). Urine was collected using a 10F silicone Foley urethral catheter with a 3 cc balloon (Tyco Healthcare Group, Mansfield, MA) and volume was recorded at baseline and at the end of the 4-hr experimental period. Calculated parameters included the following:
Arterial O2 content (ArO2) = (SaO2 ∗ 1.34 ∗ Hb ∗ .01) + (PaO2 ∗ .003) = ml/dl
Venous O2 content (VeO2) = (SvO2 ∗ 1.34 ∗ Hb ∗ .01) + (PvO2 ∗ .003) = ml/dl
Delivery of O2 (DO2) = (CO ∗ ArO2 ∗ 10) = ml/min
O2 consumption (VO2) = CO ∗ (ArO2 − VeO2) ∗ 10 = ml/min
O2 extraction ratio (O2ER) = (VO2/DO2)
Systemic vascular resistance (SVR) = (MAP − CVP)/(CO/80) = PRU
Dynamic lung compliance = Tidal Volume / [Airway Pressure (inspired) − airway pressure (expired)] = L/cmH2O
After baseline data and blood collection, hemorrhage was started at 20 ml/kg over 4 min and 40 sec to mimic the bleeding profile of an uncontrolled hemorrhage as described previously.18 Following hemorrhage, the animal was allowed to stabilize for 25 min, approximating the time spent on the battlefield from injury and hemorrhage until pre-hospital hypotensive resuscitation fluid can be given. At 30 min, a second hemorrhage of 8 ml/kg over 4 min and 40 sec was begun and resuscitation was initiated at 1.5 ml/kg/min to achieve and maintain a systolic blood pressure of 80 ± 5 mm Hg. None of the shed blood was re-infused. Animals that survived to the end of the 4-hr experimental period were euthanized with an accepted intravenous veterinary euthanasia solution (Fatal Plus, Dearborn, MI). The time of death of those animals that did not survive the 4-hr period was documented. At necropsy, consolidation of lung tissue in some animals was noted. Samples were collected and fixed in 10% buffered formaldehyde for evaluation by a veterinary pathologist.
Weight loss and fever are two common symptoms of pneumonia. Therefore, pig weight and temperature along with spleen weight, survival time, urine output during the study, and urine output/survival time were compared between the pneumonia and non-pneumonia swine. In addition, plasma levels of IL-1β and IL-10 cytokines were determined using swine immunoassay kits (Catalog # KSC0012/KSC0011 and KSC0102/KSC0101, Invitrogen Corp., Carlsbad, CA) at baseline and at 30, 60, 120 and 180 min following the start of hemorrhage. The level of detection for IL-1β and IL-10 were < 15 pg/ml and < 3 pg/ml, respectively.
Statistical AnalysisTime effects were analyzed using a two-way ANOVA with repeated measures using a Tukey adjustment when appropriate. Continuous variables were compared via the Wilcoxon test for nonparametric and score data, and a Student T-test for parametric data. All tests for significance were two-tailed, with a level of α = 0.05.
RESULTSThere were no significant differences in average pig weight or temperature at the beginning of the study or spleen weight between the two groups (Table 1).
Epidemiological control data of non-pneumonia and pneumonia groups of animals
| Pig wt. (kg) | Temp. (°C) | Spleen wt. (gm) | Survival Time (min) | Survival Rate (240 min) | Urine (mL) | Urine/Survival Time (mL/min) | |
|---|---|---|---|---|---|---|---|
| Non-pneumonia | |||||||
| n = 10 | 40.2 ± 0.42 | 37.8 ± 0.15 | 255.5 ± 16.5 | 237.4 ± 2 | 8/10 (80%) | 491 ± 158.6 | 2.06 ± 0.66 |
| Pneumonia | |||||||
| n = 5 | 39.2 ± 1.12 | 38.1 ± 0.2 | 212.6 ± 17.7 | 187.6 ± 32.8∗ | 2/5 (40%) | 53 ± 4.4∗ | 0.32 ± 0.06∗ |
| p value | 0.297 | 0.207 | 0.132 | 0.045 | 0.251 | 0.0001 | 0.0001 |
Data are mean ± SEM;∗ indicates a significant difference between the two groups.
The five pneumonia swine had histologic findings of chronic, active, diffuse or multifocal broncho-interstitial pneumonia ranging from moderate to severe, which is histologically consistent with Mycoplasma hyopneumoniae.
Survival and urine outputAll of the animals survived the first and second hemorrhage and the initial resuscitation. However, only two of the five pneumonia swine (40%) survived for the full 240 min of the study, whereas eight of the ten non-pneumonia swine (80%) survived for 240 min (p < 0.05; Table 1). Urine output and urine output corrected for survival time were significantly reduced in the pneumonia swine (p < 0.0001).
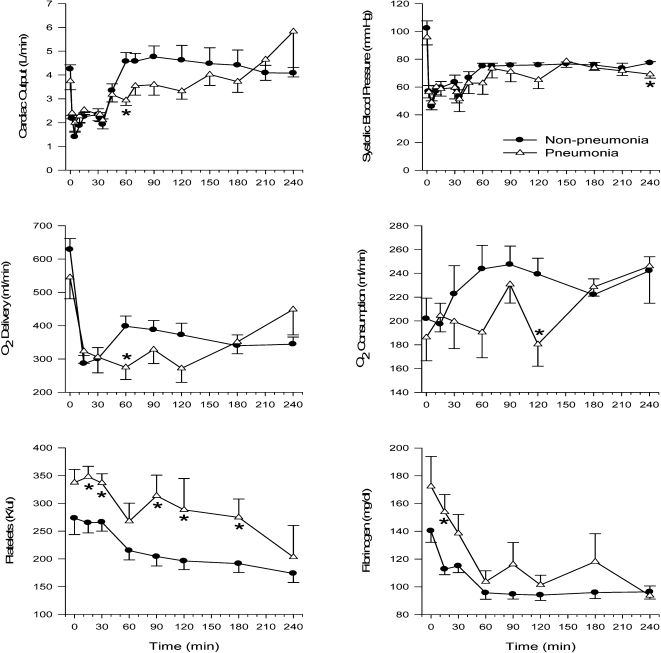
Hemodynamic dataMean systolic blood pressure and mean cardiac output were significantly lower during resuscitation in the pneumonia swine only at 240 min and 60 min, respectively (Fig 1). However, CO tended to be lower through 120 min in swine with pneumonia. There were no significant differences in arterial diastolic, mean or pulse pressure, pulmonary artery pressure, central venous pressure or heart rate between the two groups at any time (data not shown).
Respiratory and metabolic dataThere was a characteristic trend toward metabolic acidosis during and following hemorrhage, with a decrease in HCO3, base excess and pH and an increase in lactate; however, a significant difference in these parameters was not observed between the two groups of animals (Table 2). Plasma glucose concentrations rose within 15 min after hemorrhage and then fell below baseline levels after resuscitation in both groups. There were no statistically significant differences between the groups at any time. In addition, arterial and venous PO2, SO2 and PCO2 were similar between the two animal groups throughout the experimental period (data not shown). The inspired airway pressure tended to be higher in the pneumonia group (Table 2), but the difference was not significant. The calculated lung compliance also tended to be lower in the pneumonia group (0.0152 ± 0.001 in the pneumonia group compared to 0.0173 ± 0008 L/ cmH2O in the controls at baseline), but the difference was not significant and there were no changes during the course of the experiment (airway pressure only shown in Table 2).
Baseline and Post-Hemorrhage Data
| Baseline | 15 min | 30 min | 60 min | 90 min | 120 min | 180 min | 240 min | ||
|---|---|---|---|---|---|---|---|---|---|
| HCO3 (mmol/L) | NP | 29.5 ± 0.4 | 26.9 ± 0.9 | 26.8 ± 0.9 | 25.0 ± 1.1 | 24.5 ± 1.2 | 24.5 ± 1.5 | 22.8 ± 1.4 | 20.5 ± 2.1 |
| P | 30.1 ± 1.1 | 28.7 ± 1.0 | 27.4 ± 1.4 | 24.9 ± 2.3 | 23.3 ± 3.3 | 24.5 ± 2.7 | 24.1 ± 3.9 | 22.8 ± 4.8 | |
| BE (mmol/L) | NP | 5.2 ± 0.4 | 2.3 ± 1.0 | 2.4 ± 0.9 | -0.2 ± 1.4 | -0.5 ± 1.4 | -0.5 ± 1.6 | -2.0 ± 1.6 | -3.7 ± 1.9 |
| P | 5.3 ± 0.9 | 4.4 ± 0.9 | 3.4 ± 1.2 | -0.1 ± 2.5 | -1.6 ± 3.3 | -0.2 ± 2.9 | -0.6 ± 3.7 | -1.8 ± 4.5 | |
| Lactate (mmol/L) | NP | 3.1 ± 0.2 | 3.7 ± 0.7 | 4.3 ± 0.8 | 7.0 ± 1.2 | 7.6 ± 1.2 | 7.8 ± 1.1 | 9.0 ± 1.2 | 10.3 ± 1.5 |
| P | 2.4 ± 0.2 | 2.8 ± 0.3 | 3.0 ± 0.3 | 7.1 ± 1.4 | 8.5 ± 1.8 | 7.8 ± 1.6 | 9.1 ± 2.5 | 11.0 ± 3.4 | |
| pH | NP | 7.44 ± 0.01 | 7.40 ± 0.02 | 7.41 ± 0.01 | 7.35 ± 0.02 | 7.36 ± 0.02 | 7.36 ± 0.02 | 7.35 ± 0.02 | 7.39 ± 0.03 |
| P | 7.42 ± 0.03 | 7.42 ± 0.02 | 7.43 ± 0.01 | 7.35 ± 0.03 | 7.35 ± 0.02 | 7.36 ± 0.03 | 7.37 ± 0.02 | 7.35 ± 0.04 | |
| Glucose (mmol/L) | NP | 5.5 ± 0.4 | 6.8 ± 0.7 | 5.7 ± 0.7 | 3.8 ± 0.8 | 3.3 ± 0.7 | 3.4 ± 0.7 | 3.6 ± 0.9 | 2.1 ± 0.4 |
| P | 5.2 ± 0.2 | 6.2 ± 0.8 | 5.0 ± 1.2 | 4.4 ± 1.1 | 3.7 ± 1.2 | 3.0 ± 1.2 | 2.3 ± 1.2 | 3.0 ± 1.2 | |
| Hct (%) | NP | 34.8 ± 0.6 | 28.9 ± 0.8 | 29.0 ± 0.7 | 19.4 ± 1.0 | 18.2 ± 0.8 | 18.2 ± 0.8 | 17.7 ± 0.8 | 15.9 ± 1.1 |
| P | 34.7 ± 0.9 | 29.4 ± 1.7 | 29.5 ± 1.6 | 21.1 ± 2.9 | 20.0 ± 2.8 | 20.6 ± 2.6 | 21.3 ± 1.4 | 15.7 ± 2.8 | |
| Hb (gm/dL) | NP | 11.0 ± 0.2 | 9.5 ± 0.2 | 9.7 ± 0.2 | 6.6 ± 0.3 | 6.2 ± 0.2 | 6.1 ± 0.2 | 5.9 ± 0.2 | 5.5 ± 0.3 |
| P | 11.0 ± 0.4 | 9.5 ± 0.6 | 9.5 ± 0.5 | 7.0 ± 0.9 | 6.7 ± 0.9 | 6.1 ± 1.0 | 6.1 ± 1.0 | 5.4 ± 0.8 | |
| WBC 103/µL | NP | 18.7 ± 1.7 | 16.2 ± 1.8 | 17.4 ± 2.1 | 20.3 ± 2.7 | 22.4 ± 2.4 | 22.1 ± 2.3 | 22.8 ± 2.5 | 21.0 ± 3.1 |
| P | 22.9 ± 4.6 | 19.1 ± 4.4 | 19.7 ± 3.9 | 19.4 ± 3.5 | 23.9 ± 4.4 | 26.4 ± 6.7 | 32.6 ± 7.3 | 21.6 ± 8.5 | |
| PawI cm H20 | NP | 19.3 ± 0.8 | 19.1 ± 0.8 | 19.0 ± 0.8 | 17.8 ± 1.0 | 18.3 ± 0.9 | 19.3 ± 1.0 | 18.8 ± 1.4 | 19.6 ± 1.5 |
| P | 20.4 ± 2.1 | 21.2 ± 1.9 | 22.0 ± 2.3 | 20.8 ± 1.9 | 21.0 ± 2.1 | 21.0 ± 2.9 | 20.3 ± 4.8 | 22.0 ± 4.4 | |
| INR Sec | NP | 1.0 ± 0.03 | 1.05 ± 0.02 | 1.0 ± 0.02 | 1.12 ± 0.03 | 1.16 ± 0.04 | 1.15 ± 0.03 | 1.15 ± 0.04 | 1.20 ± 0.03 |
| P | 0.97 ± 0.01 | 1.02 ± 0.03 | 1.0 ± 0.02 | 1.15 ± 0.08 | 1.06 ± 0.03 | 1.20 ± 0.13 | 1.09 ± 0.03 | 1.24 ± 0.09 | |
| Creatinine mg/dL | NP | 1.27 ± 0.05 | 1.75 ± 0.13 | 1.69 ± 0.09 | 1.75 ± 0.15 | 1.63 ± 0.11 | 1.59 ± 0.12 | 1.57 ± 0.15 | 1.6 ± 0.14 |
| P | 1.22 ± 0.1 | 1.68 ± 0.21 | 1.64 ± 0.13 | 1.8 ± 0.31 | 1.84 ± 0.23 | 1.6 ± 0.18 | 1.7 ± 0.27 | 1.9 ± 0.32 |
Data are mean ± SEM; NP = non-pneumonia (n = 10); P = pneumonia (n = 5); BE = base excess; PawI = Inspiratory airway pressure; INR = International Normalized Ratio. Although there were significant upward or downward trends in some of the parameters, there were no significant differences between the two groups for any parameter at any time point.
Mean Hct, Hb, ArO2 and VeO2 decreased similarly in both groups during hemorrhage and resuscitation (Table 2). Mean DO2 and VO2 were significantly lower in the pneumonia group at 60 min and 120 min, respectively, and tended to be lower throughout this time period (Fig 1). O2 ER was not different between the two groups.
Cellular integrityMean LDH was significantly higher (p<0.05) only at 90 min in the pneumonia swine compared to non-pneumonia animals (343 ± 33.1 vs. 260 ± 14.8 U/L, respectively); LDH in both groups rose sharply at 120, 180 and 240 min, as did AST and CK. Creatinine increased at 15 min in both groups and remained elevated throughout the study. Mean total protein, amylase and ALT decreased similarly in both animal groups with the greatest decrease observed after the 30-min bleed and resuscitation (dilution effect; data not shown).
WBCThere was no significant difference in the total or differential WBC count between the groups (Table 2). The mean WBC count decreased during the first 15 min in both groups and then returned to baseline levels through the rest of the study. Neutrophils decreased slightly through 15 and 30 min in both groups and then increased steadily throughout the remainder of the study, whereas lymphocytes increased slightly for 30 min in both groups and then decreased throughout the remainder of the study (data not shown).
CoagulationPlatelets were significantly higher in the pneumonia group at 15, 30, 90, 120 and 180 min, and fibrinogen was significantly higher at 15 min (Fig 1) compared to the healthy pigs. However, there was no significant difference in PT, aPTT or INR between the two groups.
CytokinesThere was no difference in IL-1β or IL-10 levels at baseline between non-pneumonia and pneumonia animals. However, the IL-1β level in pneumonia swine was significantly (p < 0.05) elevated at 180 min compared to that in non-pneumonia swine (25.3 ± 5.7 vs. 10.2 ± 1.4 pg/mL, respectively).
DISCUSSIONA retrospective investigation of prospectively collected data has demonstrated that asymptomatic disease such as sub-clinical pneumonia significantly influenced the outcome of this study. Although it might not be surprising that these animals are more susceptible to hemorrhagic shock, only anecdotal reports have circulated and we could not find previous published reports relevant to this issue. The most significant findings were a reduced urine output and early death of the pneumonia swine. The finding of low urine output and lower cardiac output may indicate that the swine in the pneumonia group were relatively more dehydrated, or that they had an increased sensitivity to hemorrhage. There were no clinical signs of overt dehydration at baseline, such as elevated total protein concentration or differences in plasma creatinine levels. Benson et al. found that there were no differences in plasma volume in normal versus convalescent patients, despite there being indications of hypodynamic responses in the patients with pneumonia.19,20 Kumar et al. found that when patients with pneumonia were volume expanded with 400–500 ml of low molecular weight dextran, a subset showed a reduced elevation in cardiac output than when the patients were measured again upon convalescence. They concluded that these patients had depressed myocardial function, although they did not suggest a mechanism for this. It should be noted that the animals used in the current study received resuscitation after 30 minutes. Therefore, the observation that cardiac output did not rise in this group compared to that of the normal animals may indicate that their myocardial function was depressed. The lower cardiac output following hemorrhage in the pneumonia swine was responsible for the lower oxygen delivery. Lower oxygen delivery may have contributed to the higher death rate in the pneumonia swine given that the accumulated oxygen debt has been shown to be highly correlated with survival.21
Pneumonia can be a serious threat to normal pulmonary function. Various types of pneumonia are very common in the swine. Mycoplasmal pneumonia is the most prevalent form and it is usually complicated by infection with other bacteria, viruses or nematodes.12 Mycoplasmal pneumonia generally has a gradual onset and spreads slowly. The usual clinical symptom is a chronic, non-productive cough, but cough was not observed in the swine that were later diagnosed with pneumonia. Occasionally, spontaneous regression of uncomplicated pneumonia in swine has occurred, suggesting that swine have the immunologic ability to suppress the disease without medication. Thus, lesions may heal, leaving minimal evidence of pneumonia at necropsy or slaughter.
The lung, like many organ systems, has a degree of reserve capacity that is usually utilized only during extreme situations (e.g., vigorous exercise). However, the reserve capacity is threatened or reduced by disease or injury. These pneumonia swine did not show any clinical signs of pneumonia before the study, and there were no significant differences in blood PO2, PCO2, bicarbonate, base excess or pH between the two groups during the experiment with 50% oxygen ventilation. Moreover, histologic evidence of moderate-to-severe pneumonia without a corresponding increase in the total or differential WBC count suggests that the degree of lung involvement was below the threshold for clinical symptoms (sub-clinical). It is apparent that the animals were able to compensate for the existing disease when unstressed. In addition, there was no difference in the baseline levels of IL-1β and IL-10 between the non-pneumonia and pneumonia swine, suggesting that there was no existing inflammatory process in the pneumonia swine and that the animals had adapted to the pneumonia with regard to expression of these two cytokines. The significant increase in IL-1β at 180 min could indicate an increased sensitivity of the pneumonia animals to the experimental stress.
The finding of higher platelet count and fibrinogen concentration, with an elevated trend even at baseline, is consistent with the presence of infection.22 Griessenhammer et al. found that infection was the second most common cause of thrombocytosis in a study of 732 patients who were found to have an elevated (>500 × 109 L-1) platelet count in their hospital. These subgroup of patients represent 21% of all patients, and of those, 30.5% had pneumonia. The observed elevation in fibrinogen, a known acute phase protein, was comparable to the elevation found in pigs that were found at slaughter to have pneumonia.23
Limitations of studyGiven that pneumonia was not an expected result, there was no provision in the protocol for routine histopathology on all tissue. As lung tissue from the control animals appeared normal, only a few samples were sent for histological analysis after pneumonia was recognized. Therefore, histopathology data are incomplete.
ConclusionThe differences in the physiologic responses to hemorrhage between the healthy and the sub-clinically sick swine were subtle, but the presence of pneumonia reduced the survival time by impairing an animal's ability to mount as vigorous a compensatory response as healthy animals. Although few statistically significant differences in hemodynamic, metabolic and blood chemistry variables were noted between groups, this study was not powered for any of these endpoints. Thus, trends noted in this study are important as well. These data may have a clinical correlate to the trauma patient who arrives with subclinical chronic infection, does not respond to resuscitation as expected and may require further intervention to improve their outcome. Based on the obtained results, none of the data appeared predictive of an underlying pathology until after the experiment was underway.
Pneumonia is a potential problem, even in SPF animals. Therefore, every effort should be made to identify the disease before experimental investigations are started. CT scanning is an excellent technique for identifying preexisting disease. However, CT scanning is expensive and is not available in every laboratory for prescreening animals prior to experimentation. Although performed after the fact, we suggest extensive necropsy of every animal be performed to identify existing disease. In addition, we recommend developing a relationship with the vendor to compile a health history of animals, so that in the case of diseased animals from specific vendors, the investigator can work with the vendor to address the problem or have the option to change vendors if the disease cannot be eliminated or significantly reduced.
We wish to thank personnel of the Veterinary Sciences Division for their support in the maintenance and preparation of the animals during these investigations; John A. Jones for the statistical analysis, Johnny Barr for technical assistance and Briza Horace for assistance with preparation of the manuscript.
This study was supported by the U.S. Army Medical Research and Materiel Command. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Dr. Burns is an employee of Premier Consulting and Management Services (PCMS), Atlanta, GA, under contract with the U.S. Army Institute of Surgical Research.
Author Contributions: JLS, MAD conceived and designed the experiments. JLS, MDP, MAD performed the experiments. JLS, JSE, MAD, JWB evaluated and analyzed the data. JWB, JLS, JSE, MAD wrote the manuscript.